Depo-Provera
Depo-Provera medroxyprogesteroneacetate injectablesuspension, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- DEPO-PROVERA DESCRIPTION
- ACTIONS
- INDICATIONS AND USES
- DEPO-PROVERA CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DEPO-PROVERA ADVERSE REACTIONS
- DEPO-PROVERA DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PATIENT INFORMATION
- PRINCIPAL DISPLAY PANEL - 2.5 mL Vial Label
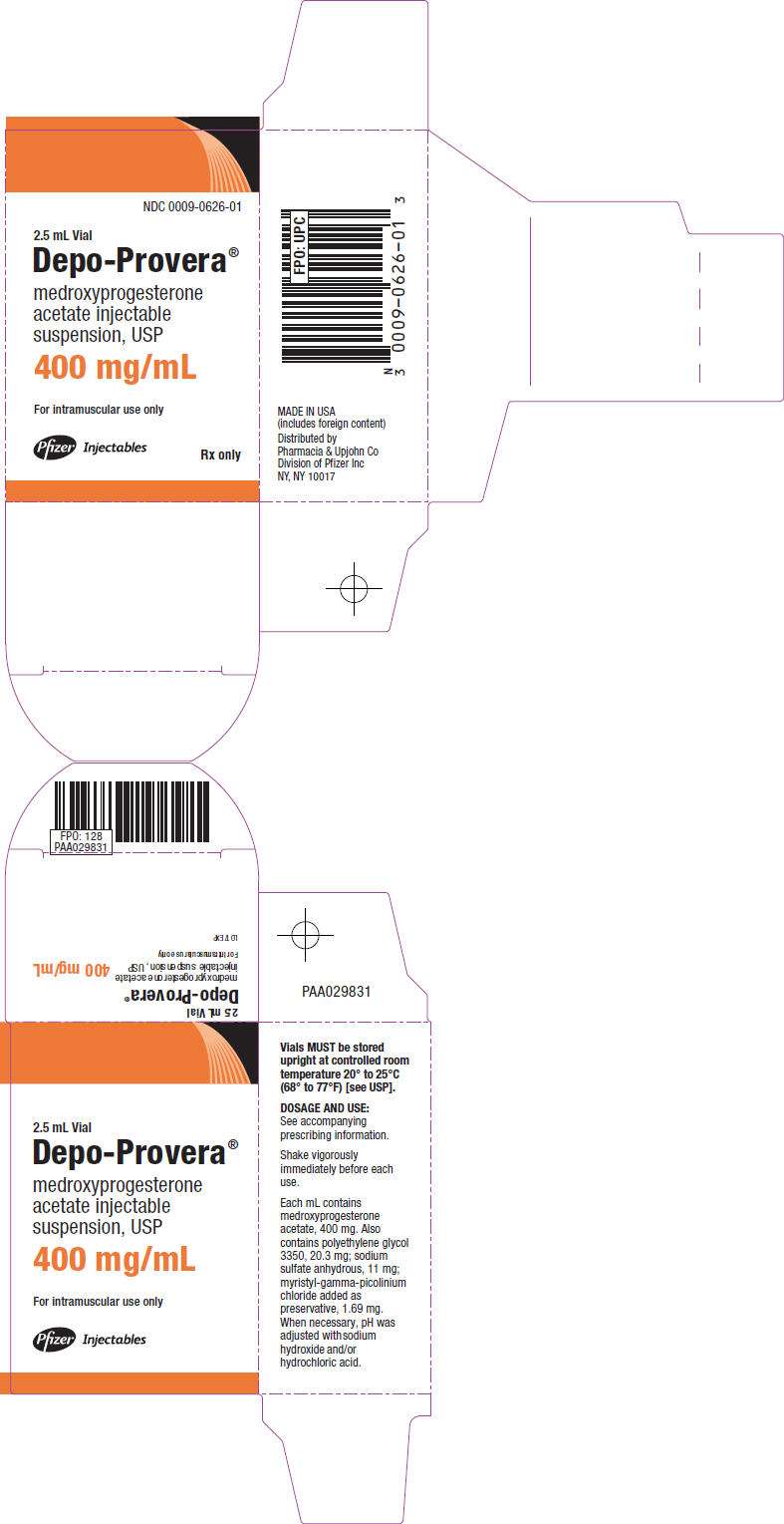
- PRINCIPAL DISPLAY PANEL - 2.5 mL Vial Carton
FULL PRESCRIBING INFORMATION
DEPO-PROVERA DESCRIPTION
DEPO-PROVERA Sterile Aqueous Suspension contains medroxyprogesterone acetate, which is a derivative of progesterone and is active by the parenteral and oral routes of administration. It is a white to off-white, odorless crystalline powder, stable in air, melting between 200° and 210° C. It is freely soluble in chloroform, soluble in acetone and in dioxane, sparingly soluble in alcohol and methanol, slightly soluble in ether and insoluble in water.
The chemical name for medroxyprogesterone acetate is Pregn-4-ene-3,20-dione, 17-(acetyloxy)-6-methyl-, (6α)-. The structural formula is:

DEPO-PROVERA for intramuscular injection is available as 400 mg/mL medroxyprogesterone acetate. Each mL of the 400 mg/mL suspension contains:
Medroxyprogesterone acetate .............400 mg
Polyethylene glycol 3350.....................20.3 mg
Sodium sulfate anhydrous ......................11 mg
with
Myristyl-gamma-picolinium
chloride ............................................1.69 mg
added as preservative
When necessary, pH was adjusted with sodium hydroxide and/or hydrochloric acid.
ACTIONS
Medroxyprogesterone acetate, administered parenterally in the recommended doses to women with adequate endogenous estrogen, transforms proliferative endometrium into secretory endometrium.
Medroxyprogesterone acetate inhibits (in the usual dose range) the secretion of pituitary gonadotropin which, in turn, prevents follicular maturation and ovulation.
Because of its prolonged action and the resulting difficulty in predicting the time of withdrawal bleeding following injection, medroxyprogesterone acetate is not recommended in secondary amenorrhea or dysfunctional uterine bleeding. In these conditions oral therapy is recommended.
INDICATIONS AND USES
Adjunctive therapy and palliative treatment of inoperable, recurrent, and metastatic endometrial or renal carcinoma.
DEPO-PROVERA CONTRAINDICATIONS
- Known or suspected pregnancy or as a diagnostic test for pregnancy
- Undiagnosed vaginal bleeding
- Known or suspected malignancy of breast
- Active thrombophlebitis, or current or past history of thromboembolic disorders, or cerebral vascular disease
- Liver dysfunction or disease
- Known sensitivity to DEPO-PROVERA (medroxyprogesterone acetate or any of its other ingredients).
WARNINGS
1. Pregnancy
The use of progestational drugs during the first four months of pregnancy is not recommended. Progestational agents have been used beginning with the first trimester of pregnancy in attempts to prevent abortion but there is no evidence that such use is effective. Furthermore, the use of progestational agents, with their uterine-relaxant properties, in patients with fertilized defective ova may cause a delay in spontaneous abortion.
2. Intrauterine Exposure
Several reports suggest an association between intrauterine exposure to progestational drugs in the first trimester of pregnancy and genital abnormalities in male and female fetuses. The risk of hypospadias (5 to 8 per 1,000 male births in the general population) may be approximately doubled with exposure to these drugs. There are insufficient data to quantify the risk to exposed female fetuses, but insofar as some of these drugs induce mild virilization of the external genitalia of the female fetus, and because of the increased association of hypospadias in the male fetus, it is prudent to avoid the use of these drugs during the first trimester of pregnancy.
If the patient is exposed to DEPO-PROVERA Sterile Aqueous Suspension during the first four months of pregnancy or if she becomes pregnant while taking this drug, she should be apprised of the potential risks to the fetus.
3. Thromboembolic Disorders
The physician should be alert to the earliest manifestations of thrombotic disorder (thrombophlebitis, cerebrovascular disorder, pulmonary embolism, and retinal thrombosis). Should any of these occur or be suspected, the drug should be discontinued immediately.
4. Ocular Disorders
Medication should be discontinued pending examination if there is a sudden partial or complete loss of vision, or if there is a sudden onset of proptosis, diplopia or migraine. If examination reveals papilledema or retinal vascular lesions, medication should be withdrawn.
5. Lactation
Detectable amounts of drug have been identified in the milk of mothers receiving progestational drugs. The effect of this on the nursing infant has not been determined.
6. Multi-dose Use
Multi-dose use of DEPOPROVERA Sterile Aqueous Suspension from a single vial requires special care to avoid contamination. Although initially sterile, any multi-dose use of vials may lead to contamination unless strict aseptic technique is observed.
PRECAUTIONS
1. Physical Examination
It is good medical practice for all women to have annual history and physical examinations, including women using DEPO-PROVERA Sterile Aqueous Suspension. The physical examination, however, may be deferred until after initiation of DEPO-PROVERA if requested by the woman and judged appropriate by the clinician. The physical examination should include special reference to blood pressure, breasts, abdomen and pelvic organs, including cervical cytology and relevant laboratory tests. In case of undiagnosed, persistent or recurrent abnormal vaginal bleeding, appropriate measures should be conducted to rule out malignancy. Women with a strong family history of breast cancer or who have breast nodules should be monitored with particular care.
2. Fluid Retention
Because progestational drugs may cause some degree of fluid retention, conditions which might be influenced by this condition, such as epilepsy, migraine, asthma, cardiac or renal dysfunction, require careful observation.
3. Vaginal Bleeding
In cases of breakthrough bleeding, as in all cases of irregular bleeding per vaginum, nonfunctional causes should be borne in mind and adequate diagnostic measures undertaken.
4. Depression
Patients who have a history of psychic depression should be carefully observed and the drug discontinued if the depression recurs to a serious degree.
5. Masking of Climacteric
The age of the patient constitutes no absolute limiting factor although treatment with progestin may mask the onset of the climacteric.
6. Use with Estrogen
Studies of the addition of a progestin product to an estrogen replacement regimen for seven or more days of a cycle of estrogen administration have reported a lowered incidence of endometrial hyperplasia. Morphological and biochemical studies of endometrial suggest that 10–13 days of a progestin are needed to provide maximal maturation of the endometrium and to eliminate any hyperplastic changes. Whether this will provide protection from endometrial carcinoma has not been clearly established.
There are possible risks which may be associated with the inclusion of progestin in estrogen replacement regimen, including adverse effects on carbohydrate and lipid metabolism. The dosage used may be important in minimizing these adverse effects.
A decrease in glucose tolerance has been observed in a small percentage of patients on estrogen-progestin combination treatment. The mechanism of this decrease is obscure. For this reason, diabetic patients should be carefully observed while receiving such therapy.
7. Prolonged Use
The effect of prolonged use of DEPO-PROVERA Sterile Aqueous Suspension at the recommended doses on pituitary, ovarian, adrenal, hepatic, and uterine function is not known.
8. Multi-dose Use
When multi-dose vials are used, special care to prevent contamination of the contents is essential. There is some evidence that benzalkonium chloride is not an adequate antiseptic for sterilizing DEPO-PROVERA Sterile Aqueous Suspension multi-dose vials. A povidone-iodine solution or similar product is recommended to cleanse the vial top prior to aspiration of contents. (See WARNINGS)
DRUG INTERACTIONS
Aminoglutethimide administered concomitantly with DEPO-PROVERA Sterile Aqueous Suspension may significantly depress the serum concentrations of medroxyprogesterone acetate. DEPO-PROVERA users should be warned of the possibility of decreased efficacy with the use of this or any related drugs.
LABORATORY TEST INTERACTIONS
The pathologist should be advised of progestin therapy when relevant specimens are submitted. The following laboratory tests may be affected by progestins including DEPO-PROVERA Sterile Aqueous Suspension:
-
a) Plasma and urinary steroid levels are decreased (e.g. progesterone, estradiol, pregnanediol, testosterone, cortisol). -
b) Gonadotropin levels are decreased. -
c) Sex-hormone binding globulin concentrations are decreased. -
d) Protein bound iodine and butanol extractable protein bound iodine may increase. T3 uptake values may decrease. -
e) Coagulation test values for prothrombin (Factor II), and Factors VII, VIII, IX, and X may increase. -
f) Sulfobromophthalein and other liver function test values may be increased. -
g) The effects of medroxyprogesterone acetate on lipid metabolism are inconsistent. Both increases and decreases in total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol have been observed in studies.
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
Long-term intramuscular administration of Medroxyprogesterone acetate (MPA) has been shown to produce mammary tumors in beagle dogs. There is no evidence of a carcinogenic effect associated with the oral administration of MPA to rats and mice. Medroxyprogesterone acetate was not mutagenic in a battery of in vitro or in vivo genetic toxicity assays.
Medroxyprogesterone acetate at high doses is an anti-fertility drug and high doses would be expected to impair fertility until the cessation of treatment.
INFORMATION FOR THE PATIENT
See Patient Information at end of insert.
DEPO-PROVERA ADVERSE REACTIONS
–(See WARNINGS for possible adverse effects on the fetus)
–breakthrough bleeding
–spotting
–change in menstrual flow
–amenorrhea
–headache
–nervousness
–dizziness
–edema
–change in weight (increase or decrease)
–changes in cervical erosion and cervical secretions
–cholestatic jaundice, including neonatal jaundice
–breast tenderness and galactorrhea
–skin sensitivity reactions consisting of urticaria, pruritus, edema and generalized rash
–acne, alopecia and hirsutism
–rash (allergic) with and without pruritis
–anaphylactoid reactions and anaphylaxis
–mental depression
–pyrexia
–fatigue
–insomnia
–nausea
–somnolence
In a few instances there have been undesirable sequelae at the site of injection, such as residual lump, change in color of skin, or sterile abscess.
A statistically significant association has been demonstrated between use of estrogen-progestin combination drugs and pulmonary embolism and cerebral thrombosis and embolism. For this reason patients on progestin therapy should be carefully observed. There is also evidence suggestive of an association with neuro-ocular lesions, e.g. retinal thrombosis and optic neuritis.
The following adverse reactions have been observed in patients receiving estrogen-progestin combination drugs:
–rise in blood pressure in susceptible individuals
–premenstrual syndrome
–changes in libido
–changes in appetite
–cystitis-like syndrome
–headache
–nervousness
–fatigue
–backache
–hirsutism
–loss of scalp hair
–erythema multiforma
–erythema nodosum
–hemorrhagic eruption
–itching
–dizziness
The following laboratory results may be altered by the use of estrogen-progestin combination drugs:
–increased sulfobromophthalein retention and other hepatic function tests
–coagulation tests: increase in prothrombin factors VII, VIII, IX, and X
–metyrapone test
–pregnanediol determinations
–thyroid function: increase in PBI, and butanol extractable protein bound iodine and decrease in T3 uptake values
DEPO-PROVERA DOSAGE AND ADMINISTRATION
The suspension is intended for intramuscular administration only.
Endometrial or renal carcinoma— doses of 400 mg to 1000 mg of DEPO-PROVERA Sterile Aqueous Suspension per week are recommended initially. If improvement is noted within a few weeks or months and the disease appears stabilized, it may be possible to maintain improvement with as little as 400 mg per month. Medroxyprogesterone acetate is not recommended as primary therapy, but as adjunctive and palliative treatment in advanced inoperable cases including those with recurrent or metastatic disease.
When multi-dose vials are used, special care to prevent contamination of the contents is essential (See WARNINGS).
HOW SUPPLIED
DEPO-PROVERA Sterile Aqueous Suspension is available as 400 mg/mL in 2.5 mL vials.
The text of the patient insert for progestational drugs is set forth below.
PATIENT INFORMATION
DEPO-PROVERA Sterile Aqueous Suspension is a progestational drug. The information below is required by the U.S. Food and Drug Administration to be provided to all patients taking such products. This information relates only to the risk to the unborn child associated with use of progestational drugs during pregnancy. For further information on the use, side effects, and other risks associated with this product, ask your doctor.
WARNING FOR WOMEN
Progesterone or progesterone-like drugs have been used to prevent miscarriage in the first few months of pregnancy. No adequate evidence is available to show that they are effective for this purpose. Furthermore, most cases of early miscarriage are due to causes which could not be helped by these drugs.
There is an increased risk of minor birth defects in children whose mothers take this drug during the first 4 months of pregnancy. Several reports suggest an association between mothers who take these drugs in the first trimester of pregnancy and genital abnormalities in male and female babies. The risk to the male baby is the possibility of being born with a condition in which the opening of the penis is on the underside rather than the tip of the penis (hypospadias). Hypospadias occurs in about 5 to 8 per 1000 male births and is about doubled with exposure to these drugs. There is not enough information to quantify the risk to exposed female fetuses, but enlargement of the clitoris and fusion of the labia may occur, although rarely.
Therefore, since drugs of this type may induce mild masculinization of the external genitalia of the female fetus, as well as hypospadias in the male fetus, it is wise to avoid using the drug during the first trimester of pregnancy.
These drugs have been used as a test for pregnancy but such use is no longer considered safe because of possible damage to a developing baby. Also, more rapid methods for testing for pregnancy are now available.
If you take DEPO-PROVERA Sterile Aqueous Suspension and later find you were pregnant when you took it, be sure to discuss this with your doctor as soon as possible.
Rx only

LAB-0143-3.0
May 2006
PRINCIPAL DISPLAY PANEL - 2.5 mL Vial Label
2.5 mL Vial
NDC 0009-0626-01
Depo-Provera®
medroxyprogesterone acetate
injectable suspension, USP
400 mg/mL
For IM use only
Rx only

PRINCIPAL DISPLAY PANEL - 2.5 mL Vial Carton
NDC 0009-0626-01
2.5 mL Vial
Depo-Provera®
medroxyprogesterone
acetate injectable
suspension, USP
400 mg/mL
For intramuscular use only
Pfizer Injectables
Rx only
Depo-Proveramedroxyprogesterone acetate INJECTION, SUSPENSION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||