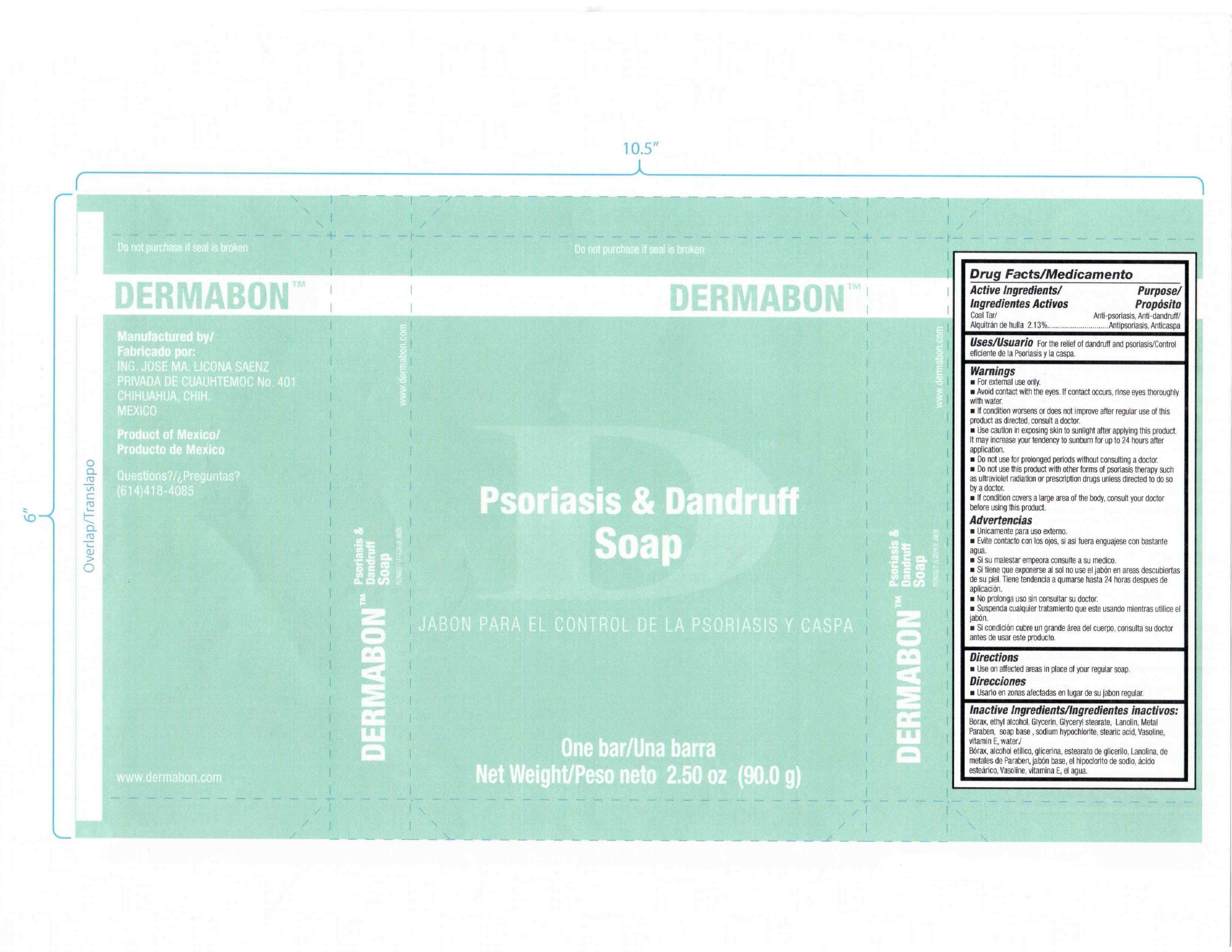
Dermabon Psoriasis and Dandruff Soap
Jose Maria Licona Saenz
Jose Maria Licona Saenz
Dermabon Psoriasis and Dandruff Soap
FULL PRESCRIBING INFORMATION
Active ingredient
Drug Facts
Active Ingredients Purpose
Coal Tar 2.13 percent Anti-psoriasis, Anti-dandruff
Purpose
Uses
For the relief of dandruff and psoriasis
Warnings
For external use only.
Avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with water.
If condition worsens or does not improve after regular use of this product as directed, consult a doctor.
Use caution in exposing skin to sunlight after applying this product. It may increase your tendency to sunburn for up to 24 hours after application.
Do not use for prolonged periods without consulting a doctor.
Do not use this product with other forms of psoriasis therapy such as ultraviolet radiation or prescription drugs unless directed to do so by a doctor.
If condition covers a large area of the body, consult your doctor before using this product.
Directions
Use on affected areas in place of your regular soap.
Inactive Ingredients
Borax, ethyl alcohol, Glycerin , Glyceryl stearate, Lanolin, Metal
Paraben, soap base, sodium hypochlorite, stearic acid , Vasoline,
vitamin E, water.
Manufactured by
ING. JOSE MA. LICONA SAENZ
PRIVADA DE CUAUHTEMOC No.401
CHIHUAHUA, CHIH.
MEXICO
Product of Mexico
Questions
614 418 4085
www.dermabon.com
Do not purchase if seal is broken
Dermabon Psoriasis and Dandruff SoapCOAL TAR SOAP
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||