Dexamethasone Sodium Phosphate
General Injectables & Vaccines, Inc.
Dexamethasone Sodium Phosphate Injection, USP 4mg/mL 5mL Multidose Vial
FULL PRESCRIBING INFORMATION: CONTENTS*
- DEXAMETHASONE SODIUM PHOSPHATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- DEXAMETHASONE SODIUM PHOSPHATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DEXAMETHASONE SODIUM PHOSPHATE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PACKAGE LABEL
FULL PRESCRIBING INFORMATION
DEXAMETHASONE SODIUM PHOSPHATE DESCRIPTION
Dexamethasone sodium phosphate is a water-soluble inorganic ester of dexamethasone. It occurs as a white or slightly yellow crystalline powder, is odorless or has a slight odor of alcohol, is exceedingly hygroscipic and is freely soluble in water.
Dexamethasone sodium phosphate is an adrenocortical steroid anti-inflammatory drug.
Chemically, dexamethasone sodium phosphate is 9-Gluoro-11B,17,21-trihydroxy-16a-methylpregna-1,4-diene-3,20-dione 21-(dihydrogen phosphate) disodium salt and has the following structural formula:
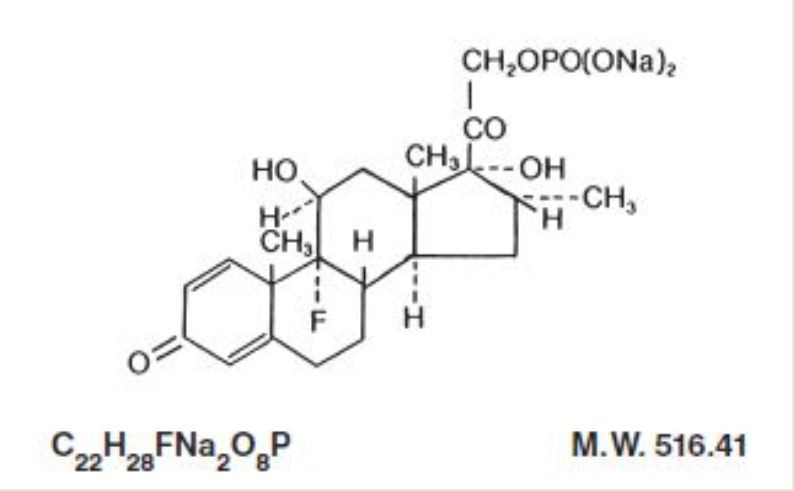
Dexamethasone Sodium Phosphate Injection, USP is a sterile solution of dexamethasone sodium phosphate in Water for Injection for intravenous (IV), intramuscular (IM), intra-articular, soft-tissue or intralesional use.
Each mL contains dexamethasone sodium phosphate equivalent to dexamethasone phosphate 4 mg or dexamethasone 3.33 mg; bensyl alcohol 10 mg added as preservative; sodium citrate dihydrate 11 mg; sodium sulfite 1 mg as antioxidant; Water for Injection q.s. Citric acid and/or sodium hydroxide may have been added for pH adjustment (7.0-8.5). Air in the container is displaced by nitrogen.
CLINICAL PHARMACOLOGY
Dexamethasone sodium phosphate has a rapid onset but short duration of action when compared with less soluble preparations. Because of this, it suitable for the treatment of acute disorders responsive to adrenocortical steroid therapy.
Naturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic analogs, including dexamethasone, are primarily used for their potent anti-inflammatory effects in dis-orders of many organ systems.
Glucocorticoids cause profound and varied metabolic effects. In addition, they modify the body's immune responses to diverse stimuli.
At equipotent anti-inflammatory doses, dexamethasone almost completely lacks the sodium-retaining property of hydrocortisone and closely related derivatives of hydrocortisone.
INDICATIONS & USAGE
Intravenous or Intramuscular Injection
When oral therapy is not feasible and the strength, dosage form, and route of administration of the drug reasonably lend the preparation to the treatment of the condition, those products labeled for intravenous or intramuscular use are indicated as follows:
*Endocrine Disorders
Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the drug of choice; synthetic analogs may be used in conjunction with mineralocorticoids where applicable; in infancy, mineralocorticoid supplementation is of particular importance) Acute adrenocortical insuggiciency (hydrocortisone or cortisone is the drug of choice; mineralocorticoid supplementation may be necessary, particularly when synthetic analogs are used) Preoperatively, and in the event of serious trauma or illness, in patients with known adrenal insufficiency or when adrenocortical reserve is doubtful Shock unresponsive to conventional therapy if adrenocortical insufficiency exists or is suspected
Congenital adrenal hyperplasia
Nonsuppurative thyroiditis
Hypercalcemia associated with cancer
*Rheumatic Disorders
As adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in:
Post-traumatic osteoarthritis
Synovitis of osteoarthritis
Rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy)
Acute and subacute bursitis
Epicondylitis
Acute nonspecific tenosynovitis
Acute gouty arthritis
Psoriatic arthritis
Ankylosing spondylitis
*Collagen Diseases
During an exacerbation or as maintenance therapy in selected cases of:
Systemic lupus erythematosus
Acute rheumatic carditis
*Dermatologic Diseases
Pemphigus
Severe erythema multiforme (Stevens-Johnson syndrome)
Exfoliative dermatitis
Bullous dermatitis herpetiformis
Severe seborrheic dermatitis
Severe psoriasis
Mycosis fungoides
*Allergic States
Control of severe or incapacitating allergic conditions intractable to adequate trials of conventional treatment in:
Bronchial asthma
Contact dermatitis
Atopic dermatitis
Serum sickness
Seasonal or perennial allergic rhinitis
Drug hypersensitivity reactions
Urticarial transfusion reactions
Acute noninfectious laryngeal edema (epinephrine is the drug of first choice)
DEXAMETHASONE SODIUM PHOSPHATE CONTRAINDICATIONS
Systemic fungal infections (see WARNINGS regarding amphotericin B). Hypersensitivity to any component of this product, including sulfites (see WARNINGS).
WARNINGS
PRECAUTIONS
This product, like many other steroid formulations, is sensitive to heat. Therefore, it should not be autoclaved when it is desirable to sterilize the exterior of the vial.
Following prolonged therapy, withdrawal of corticosteroids may result in symptoms of the corticosteroid withdrawal syndrome including fever, myalgia, arthralgia, and malaise. This may occur in patients even without evidence of adrenal insufficiency.
There is an enhanced effect of corticosteroids in patients with hypothyroidism and in those with cirrhosis.
Corticosteroids should be used cautiously in patents with ocular herpes simplex for fear of corneal perforation.
The lowest possible dose of corticosteroid should be used to control the condition under treatment, and when reduction in dosage is possible, the reduction must be gradual.
Psychic derangements may appear when corticosteroids are used, ranging from euphoria, insomnia, mood swings, personality changes, and severe depression to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated by corticosteroids.
Aspirin should be used cautiously in conjunction with corticosteroids in hypoprothrombinemia.
Steroids should be used with caution in nonspecific ulcerative colitis, if there is a probability or impending perforation, abscess, or other pyogenic infection, also in diverticulitis, fresh intestinal anastomoses, active or latent peptic ulcer, renal insufficiency, hypertension, osteoporosis, and myasthenis gravis. Signs of peritoneal irritation following gastrointestinal perforation in patients receiving large does of corticosteroids may be minimal or absent. Fat embolism has been reported as a possible complication of hypercortisonism.
When large doses are given, some authorities advise that antacids be administered between meals to help to prevent peptic ulcer.
Growth and development of infants and children on prolonged corticosteroid therapy should be carefully followed.
Steroids may increase or decrease motility and number of permatozoa in some patients.
Phenytoin, phenobarbital, ephedrine, and rifampin may enhance the metabolic clearance of corticosteroids resulting in decreased blood levels and lessened physiologic activity, thus requiring adjustment corticosteroid dosage. These interactions may interfere with dexamethasone suppression test which should be interpreted with caution during administration of these drugs.
False negative results in the dexamethasone suppression test (DST) in patients being rreated with indomethacin have been reported. Thus, results of the DST should be interpreted with caution in these patients.
The prothrombin time should be checked frequently in patients who are receiving corticosteroids and coumarin anticagulants at the same time because of reports that corticosteroids have altered the response to these anticoagulants. Studies have shown that the usual effect produced by adding corticosteroids is inhibition of response to coumarins, although there have been some conflicting reports of potentiation not substantiated by studies.
When corticosteroids are administered concomitantly with potassium-depleting diuretics, patients should be observed closely for development of hypokalemia.
Intra-articular injection of a corticosteroid may produce systemic as well as local effects.
Appropriate examination of any joint fluid present is necessary to exclude a septic process.
A marked increase in pain accompanied by local swelling, further restriction of joint motion, fever, and malaise is suggestive of septic arthritis. If this complication occurs and the diagnosis of sepsis is confirmed, appropriate antimicrobial therapy should be instituted.
Injection of a steroid into an infected site is to be avoided.
Corticosteroids should not be injected into unstable joints.
Patients should be impressed strongly with the importance of not overusing joints in which symptomatic benefit has been obtained as as the inflammatory process remains active.
Frequent intra-articular injection may result in damage to joint tissues.
The slower rate of absorption by intramuscular administration should be recognized.
Information for Patients
Persons who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chickenpox or measles. Patients should also be advised that if they are exposed, medical advice should be sought without delay.
DEXAMETHASONE SODIUM PHOSPHATE ADVERSE REACTIONS
Fluid and electrolyte disturbances:
Sodium retention
Fluid retention
Congestive heart failure in susceptible patients
Potassium loss
Hypokalemic alkalosis
Hypertension
Musculoskeletal:
Muscle weakness
Steroid myopathy
Loss of muscle mass
Osteoporosis
Pathologic fracture of lang bones
Vertebral compression fractures
Aseptic necrosis of femoral and humeral heads
Tendon rupture
Gastrointestinal:
Peptic ulcer with possible subsequent perforation and hemorrhage
Perforation of the small and large bowel, particularly in patients with inflammatory bowel disease
Pancreatitis
Abdominal distention
Ulcerative esophagitis
Dermatologic:
Impaired would healing
thin fagile skin
Petechiae and ecchymoses
Erythema
Increased sweating
May suppress reactions to skin tests
Burning or tingling, especially in the perineal area (after IV injection)
Other cutaneous reactions, such as allergic dermatitis, urticaria, angioneurotic edema
Neurologic:
Convulsions
Increased intracranial pressure with papilledema (pseudotumor cerbri) usually after treatment
Vertigo
Headache
Psychic disturbances
Endocrine:
Menstrual irregularities
Development of cushingoid state
Suppression of growth in children
Secondary adrenocortical and pituitary unresponsiveness, particularly in times of stress, as in trauma, surgery, or illness
Decreased carbohydrate tolerance
Manifestations of latent diabetes mellitus
Increased requirements for insulin or oral hypoglycemic agents in diabetics
Hirsutism
Ophthalmic:
Posterior subcapsular cataracts
Increased intraocular pressure
Glaucoma
Exophthalmos
Metabolic:
Negative nitrogen balance due to protein catabolism
Cardiovascular:
Myocardial rupture following recent myocardial infarction (see WARNINGS)
Other:
Anaphylactoid or hypersensitivity reactions
Thromboembolism
Weight gain
Increased appetite
Nausea
Malaise
Hiccups
The following additional adverse reactions are related to parenteral corticosteroid therapy:
Rare instances of blindness associated with intralesional therapy around the face and head
Hyperpigmentation or hypopigmentation
Subcutaneous and cutaneous atrophy
Sterile abscess
Post-injection flare (following intra-articular use)
Charcot-like arthropathy
OVERDOSAGE
Reports of acute toxicity and/or death following over-dosage of glucocorticoids are rare. In the event of over-dosage, no specific antidote is available; treatment is supportive and symptomatic.
The oral LD50 of dexamethasone in female mice was 6.5 g/kg. The intravenous LD50 of dexamethasone sodium phosphate in female mice was 794mg/kg.
DOSAGE & ADMINISTRATION
Dexamethasone sodium phosphate injection, 4 mg/mL-For intravenous, intramuscular, intra-articular, intralesional, and soft tissue injection.
Dexamethasone sodium phosphate injection can be given directly from the vial, or it can be added to Sodium Chloride Injection or Dextrose Injection and administered by intravenous drip.
Solutions used for intravenous administration or further dilution of this product should be preservative free when used in the neonate, especially the premature infant.
When it is mixed with an infusion solution, sterile precautions should be observed. Since infusion solutions generally do not contain preservatives, mixtures should be used within 24 hours.
DOSAGE REQUIREMENTS ARE VARIABLE AND MUST BE INDIVIDUALIZED ON THE BASIS OF THE DISEASE AND THE RESPONSE OF THE PATIENT.
Intravenous and Intramuscular Injection:
The initial dosage of dexamethasone sodium phosphate injection varies from 0.5 to 9mg a day depending on the disease being treated. In less severe diseases doses lower than 0.5 mg may suffice, while in severe diseases doses higher than 9 mg may be required.
The initial dosage should be maintained or adjusted until the patient's response is satisfactory. If a satisfactory clinical response does not occur after a reasonable period of time, discontinue dexamethasone sodium phosphate injection and transfer the patient to other therapy.
After a favorable initial response, the proper maintenance dosage should be determined by decreasing the initial dosage in small amounts to the lowest dosage that maintains an adequate clinical response.
Patients should be observed closely for signs that might require dosage adjustment, including changes in clinical status resulting from remissions or exacerbations of the disease, individuals drug responsiveness, and the effect of stress (e.g., surgery, infection, trauma). During stress it may be necessary to increase dosage temporarily.
If the drug is to be stopped after more than a few days of treatment, it usually should be withdrawn gradually.
When the intravenous route of administration is used, dosage usually should be the same as the oral dosage. In certain overwhelming, acute, life threatening situations, however, administration in dosages exceeding the usual dosages may be justified and may be in multiples of the oral dosages. The slower rate of absorption by intramuscular administration should be recognized.
Shock
There is a tendency in current medical practice to use high (pharmacologic) doses of corticosteroids for the treatment of unresponsive shock. The following dosages of dexamethasone sodium phosphate injection have been suggested by various authors:
|
|
|
Author |
Dosage |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cavanagh |
3 mg/kg of body weight per 24 hours by constant intravenous infusion after an initial intravenous injection of 20 mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Schumer |
1 mg/kg of body weight as a single intravenous injection |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dietzman |
2 to 6 mg/kg of body weight as a single intravenous injection |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Frank |
40 mg initially followed by repeat intravenous injection every 4 to 6 hours while shock persists |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Oaks |
40 mg initially followed by repeat intravenous injection every 2 to 6 hours while shock persists |
|
|
|
|
|
|
|
|
|
|
|
|
| Site of Injection | Amount of Dexamethasone Phosphate (mg) |
| Large Joints (e.g., Knee) |
2 to 4 |
| Small Joints (e.g., Interphalangeal, Temporomandibular) |
0.8 to 1 |
| Bursae |
2 to 3 |
| Tendon Sheaths |
0.4 to 1 |
| Soft Tissue Infiltration |
2 to 6 |
| Ganglia |
1 to 2 |
|
|
|
HOW SUPPLIED
| Product No. |
NDC No. |
|
| 16501 |
63323-165-01 |
Dexamethasone Sodium Phosphate Injection, USP (equivalent to dexamethasone phosphate 4 mg/mL) 2 mL flip-top vials |
| 16505 |
63323-165-05 |
Dexamethasone Sodium Phosphate Injection, USP (equivalent to dexamethasone phosphate 4mg/mL) 5 mL flip-top vials packaged in 25. |
| 16530 |
63323-165-30 |
Dexamethasone Sodium Phosphate Injection, USP (equivalent to dexamethasone phosphate 4 mg/mL) 30 mL flip-top vials packaged individually. |
REFERENCES
Cavanagh, D.; singh, K.B.; Endotoxin shock in pregnancy and abortion, in: "Corticosteroids in the Treatment of Shock", Schumer, W.; Nyhus, L.M., Editors, Urbana, University of Illinois Press, 1970, pp. 86-96
Dietzman, R.H.; Ersek, R.A.; Bloch, J.M.: Lillehei, R.C.: High-output, low-resistance gram-negative septic shock in , Angiology 20: 691-700, Dec. 1969.
Frank, E.: Clinical observations in shock and management (In: Shields, T.F., ed.: Symposium on current concepts and management of shock), J. Maine Med. Ass. 59: 195-200, Oct. 1968.
Oaks, W.W.; Cohen, H.E.: Endotoxin shock in the geriatric patient, Geriat. 22: 120-130, Mar. 1967.
Schumer, W.; Nyhus, L.M.: Corticosteroid effect on biochemical parameters of human oligemic shock, Arch. Surg. 100: 405-408, Apr. 1970.
APP
APP Pharmaceuticals, LLC
Schaumburg, IL 60173
45799E
Revised: January 2008
PACKAGE LABEL

Dexamethasone Sodium PhosphateDexamethasone Sodium Phosphate INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||