DIOVAN HCT
FULL PRESCRIBING INFORMATION
11 DESCRIPTIONDiovan HCT (valsartan and hydrochlorothiazide, USP) is a combination of valsartan, an orally active, specific angiotensin II receptor blocker (ARB) acting on the AT1 receptor subtype, and hydrochlorothiazide, a diuretic.
Valsartan, a nonpeptide molecule, is chemically described as N-(1-oxopentyl)-N-[[2′-(1H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L-Valine. Its empirical formula is C24H29N5O3, its molecular weight is 435.5, and its structural formula is

Valsartan is a white to practically white fine powder. It is soluble in ethanol and methanol and slightly soluble in water.
Hydrochlorothiazide USP is a white, or practically white, practically odorless, crystalline powder. It is slightly soluble in water; freely soluble in sodium hydroxide solution, in n-butylamine, and in dimethylformamide; sparingly soluble in methanol; and insoluble in ether, in chloroform, and in dilute mineral acids. Hydrochlorothiazide is chemically described as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide.
Hydrochlorothiazide is a thiazide diuretic. Its empirical formula is C7H8ClN3O4S2, its molecular weight is 297.73, and its structural formula is

Uses
Diovan HCT (valsartan and hydrochlorothiazide, USP) is indicated for the treatment of hypertension.
Diovan HCT may be used in patients whose blood pressure is not adequately controlled on monotherapy.
Diovan HCT may be used as initial therapy in patients who are likely to need multiple drugs to achieve blood pressure goals.
The choice of Diovan HCT as initial therapy for hypertension should be based on an assessment of potential benefits and risks.
Patients with stage 2 hypertension are at a relatively high risk for cardiovascular events (such as strokes, heart attacks, and heart failure), kidney failure, and vision problems, so prompt treatment is clinically relevant. The decision to use a combination as initial therapy should be individualized and should be shaped by considerations such as baseline blood pressure, the target goal and the incremental likelihood of achieving goal with a combination compared to monotherapy. Individual blood pressure goals may vary based upon the patient’s risk.
 CONTRAINDICATIONS
CONTRAINDICATIONSDiovan HCT (valsartan and hydrochlorothiazide, USP) is contraindicated in patients who are hypersensitive to any component of this product.
Because of the hydrochlorothiazide component, this product is contraindicated in patients with anuria or hypersensitivity to other sulfonamide-derived drugs.
Because clinical studies are conducted under widely varying conditions, adverse reactions rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Hypertension
Diovan HCT (valsartan and hydrochlorothiazide, USP) has been evaluated for safety in more than 5,700 patients, including over 990 treated for over 6 months, and over 370 for over 1 year. Adverse experiences have generally been mild and transient in nature and have only infrequently required discontinuation of therapy. The overall incidence of adverse reactions with Diovan HCT was comparable to placebo.
The overall frequency of adverse reactions was neither dose-related nor related to gender, age, or race. In controlled clinical trials, discontinuation of therapy due to side effects was required in 2.3% of valsartan-hydrochlorothiazide patients and 3.1% of placebo patients. The most common reasons for discontinuation of therapy with Diovan HCT were headache and dizziness.
The only adverse reaction that occurred in controlled clinical trials in at least 2% of patients treated with Diovan HCT and at a higher incidence in valsartan-hydrochlorothiazide (n=4372) than placebo (n=262) patients was nasopharyngitis (2.4% vs. 1.9%).
Dose-related orthostatic effects were seen in fewer than 1% of patients. In individual trials, a dose-related increase in the incidence of dizziness was observed in patients treated with Diovan HCT.
Other adverse reactions that have been reported with valsartan-hydrochlorothiazide (>0.2% of valsartan-hydrochlorothiazide patients in controlled clinical trials) without regard to causality, are listed below:
Cardiovascular: Palpitations and tachycardia
Ear and Labyrinth: Tinnitus and vertigo
Gastrointestinal: Dyspepsia, diarrhea, flatulence, dry mouth, nausea, abdominal pain, abdominal pain upper, and vomiting
General and Administration Site Conditions: Asthenia, chest pain, fatigue, peripheral edema and pyrexia
Infections and Infestations: Bronchitis, bronchitis acute, influenza, gastroenteritis, sinusitis, upper respiratory tract infection and urinary tract infection
Investigations: Blood urea increased
Musculoskeletal: Arthralgia, back pain, muscle cramps, myalgia, and pain in extremity
Nervous System: Dizziness postural, paresthesia, and somnolence
Psychiatric: Anxiety and insomnia
Renal and Urinary: Pollakiuria
Reproductive System: Erectile dysfunction
Respiratory, Thoracic and Mediastinal: Dyspnea, cough, nasal congestion, pharyngolaryngeal pain and sinus congestion
Skin and Subcutaneous Tissue: Hyperhidrosis and rash
Vascular: Hypotension
Other reported reactions seen less frequently in clinical trials included abnormal vision, anaphylaxis, bronchospasm, constipation, depression, dehydration, decreased libido, dysuria, epistaxis, flushing, gout, increased appetite, muscle weakness, pharyngitis, pruritus, sunburn, syncope, and viral infection
Valsartan: In trials in which valsartan was compared to an ACE inhibitor with or without placebo, the incidence of dry cough was significantly greater in the ACE inhibitor group (7.9%) than in the groups who received valsartan (2.6%) or placebo (1.5%). In a 129-patient trial limited to patients who had had dry cough when they had previously received ACE inhibitors, the incidences of cough in patients who received valsartan, hydrochlorothiazide, or lisinopril were 20%, 19%, 69% respectively (p less than 0.001).
Other reported reactions seen less frequently in clinical trials included chest pain, syncope, anorexia, vomiting, and angioedema.
Hydrochlorothiazide: Other adverse reactions that have been reported with hydrochlorothiazide, without regard to causality, are listed below:
Body As A Whole: weakness
Digestive: pancreatitis, jaundice (intrahepatic cholestatic jaundice), sialadenitis, cramping, gastric irritation;
Hematologic: aplastic anemia, agranulocytosis, leukopenia, hemolytic anemia, thrombocytopenia;
Hypersensitivity: purpura, photosensitivity, urticaria, necrotizing angiitis (vasculitis and cutaneous vasculitis), fever, respiratory distress including pneumonitis and pulmonary edema, anaphylactic reactions;
Metabolic: hyperglycemia, glycosuria, hyperuricemia;
Musculoskeletal: muscle spasm;
Nervous System/Psychiatric: restlessness;
Renal: renal failure, renal dysfunction, interstitial nephritis;
Skin: erythema multiforme including Stevens-Johnson syndrome, exfoliative dermatitis including toxic epidermal necrolysis;
Special Senses: transient blurred vision, xanthopsia.
Initial Therapy - Hypertension
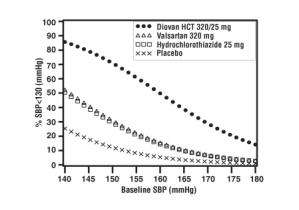
In a clinical study in patients with severe hypertension (diastolic blood pressure greater than or equal to 110 mmHg and systolic blood pressure greater than or equal to 140 mmHg), the overall pattern of adverse reactions reported through six weeks of follow-up was similar in patients treated with Diovan HCT as initial therapy and in patients treated with valsartan as initial therapy. Comparing the groups treated with Diovan HCT (force-titrated to 320/25 mg) and valsartan (force-titrated to 320 mg), dizziness was observed in 6% and 2% of patients, respectively. Hypotension was observed in 1% of those patients receiving Diovan HCT and 0% of patients receiving valsartan. There were no reported cases of syncope in either treatment group. Laboratory changes with Diovan HCT as initial therapy in patients with severe hypertension were similar to those reported with Diovan HCT in patients with less severe hypertension [See Clinical Studies (14.2) and Drug Interactions (7.3)].
The following additional adverse reactions have been reported in valsartan or valsartan/hydrochlorothiazide postmarketing experience:
Hypersensitivity: There are rare reports of angioedema;
Digestive: Elevated liver enzymes and very rare reports of hepatitis;
Renal: Impaired renal function;
Clinical Laboratory Tests: Hyperkalemia;
Dermatologic: Alopecia;
Vascular: Vasculitis;
Nervous System: Syncope.
Rare cases of rhabdomyolysis have been reported in patients receiving angiotensin II receptor blockers.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure
10 OVERDOSAGEValsartan – Hydrochlorothiazide : Limited data are available related to overdosage in humans. The most likely manifestations of overdosage would be hypotension and tachycardia; bradycardia could occur from parasympathetic (vagal) stimulation. Depressed level of consciousness, circulatory collapse and shock have been reported. If symptomatic hypotension should occur, supportive treatment should be instituted.
Valsartan is not removed from the plasma by dialysis.
The degree to which hydrochlorothiazide is removed by hemodialysis has not been established. The most common signs and symptoms observed in patients are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias.
In rats and marmosets, single oral doses of valsartan up to 1524 and 762 mg/kg in combination with hydrochlorothiazide at doses up to 476 and 238 mg/kg, respectively, were very well tolerated without any treatment-related effects. These no adverse effect doses in rats and marmosets, respectively, represent 46.5 and 23 times the maximum recommended human dose (MRHD) of valsartan and 188 and 113 times the MRHD of hydrochlorothiazide on a mg/m2 basis. (Calculations assume an oral dose of 320 mg/day valsartan in combination with 25 mg/day hydrochlorothiazide and a 60-kg patient.)
Valsartan : Valsartan was without grossly observable adverse effects at single oral doses up to 2000 mg/kg in rats and up to 1000 mg/kg in marmosets, except for salivation and diarrhea in the rat and vomiting in the marmoset at the highest dose (60 and 31 times, respectively, the maximum recommended human dose on a mg/m2 basis). (Calculations assume an oral dose of 320 mg/day and a 60-kg patient.)
Hydrochlorothiazide : The oral LD50 of hydrochlorothiazide is greater than 10 g/kg in both mice and rats, which represents 2027 and 4054 times, respectively, the maximum recommended human dose on a mg/m2 basis. (Calculations assume an oral dose of 25 mg/day and a 60-kg patient.)
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action
Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Valsartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is therefore independent of the pathways for angiotensin II synthesis.
There is also an AT2 receptor found in many tissues, but AT2 is not known to be associated with cardiovascular homeostasis. Valsartan has much greater affinity (about 20,000-fold) for the AT1 receptor than for the AT2 receptor. The primary metabolite of valsartan is essentially inactive with an affinity for the AT1 receptor about one 200th that of valsartan itself.
Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is widely used in the treatment of hypertension. ACE inhibitors also inhibit the degradation of bradykinin, a reaction also catalyzed by ACE. Because valsartan does not inhibit ACE (kininase II) it does not affect the response to bradykinin. Whether this difference has clinical relevance is not yet known. Valsartan does not bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation.
Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and angiotensin II circulating levels do not overcome the effect of valsartan on blood pressure.
Hydrochlorothiazide is a thiazide diuretic. Thiazides affect the renal tubular mechanisms of electrolyte reabsorption, directly increasing excretion of sodium and chloride in approximately equivalent amounts. Indirectly, the diuretic action of hydrochlorothiazide reduces plasma volume, with consequent increases in plasma renin activity, increases in aldosterone secretion, increases in urinary potassium loss, and decreases in serum potassium. The renin-aldosterone link is mediated by angiotensin II, so coadministration of an angiotensin II receptor antagonist tends to reverse the potassium loss associated with these diuretics.
The mechanism of the antihypertensive effect of thiazides is unknown.
12.2 PharmacodynamicsValsartan : Valsartan inhibits the pressor effect of angiotensin II infusions. An oral dose of 80 mg inhibits the pressor effect by about 80% at peak with approximately 30% inhibition persisting for 24 hours. No information on the effect of larger doses is available.
Removal of the negative feedback of angiotensin II causes a 2- to 3-fold rise in plasma renin and consequent rise in angiotensin II plasma concentration in hypertensive patients. Minimal decreases in plasma aldosterone were observed after administration of valsartan; very little effect on serum potassium was observed.
In multiple-dose studies in hypertensive patients with stable renal insufficiency and patients with renovascular hypertension, valsartan had no clinically significant effects on glomerular filtration rate, filtration fraction, creatinine clearance, or renal plasma flow.
In multiple-dose studies in hypertensive patients, valsartan had no notable effects on total cholesterol, fasting triglycerides, fasting serum glucose, or uric acid.
Hydrochlorothiazide: After oral administration of hydrochlorothiazide, diuresis begins within 2 hours, peaks in about 4 hours and lasts about 6 to 12 hours.
12.3 PharmacokineticsValsartan : Valsartan peak plasma concentration is reached 2 to 4 hours after dosing. Valsartan shows bi-exponential decay kinetics following intravenous administration, with an average elimination half-life of about 6 hours. Absolute bioavailability for the capsule formulation is about 25% (range 10%-35%). Food decreases the exposure (as measured by AUC) to valsartan by about 40% and peak plasma concentration (Cmax) by about 50%. AUC and Cmax values of valsartan increase approximately linearly with increasing dose over the clinical dosing range. Valsartan does not accumulate appreciably in plasma following repeated administration.
Hydrochlorothiazide : Thiazide diuretics are eliminated by the kidney, with a terminal half-life of 5-15 hours.
Geriatric : Exposure (measured by AUC) to valsartan is higher by 70% and the half-life is longer by 35% in the elderly than in the young. No dosage adjustment is necessary [See Dosage and Administration (2.1 )].
Gender : Pharmacokinetics of valsartan does not differ significantly between males and females.
Race : Pharmacokinetic differences due to race have not been studied.
Renal Insufficiency : There is no apparent correlation between renal function (measured by creatinine clearance) and exposure (measured by AUC) to valsartan in patients with different degrees of renal impairment. Consequently, dose adjustment is not required in patients with mild-to-moderate renal dysfunction. No studies have been performed in patients with severe impairment of renal function (creatinine clearance greater than 10 mL/min). Valsartan is not removed from the plasma by hemodialysis. In the case of severe renal disease, exercise care with dosing of valsartan [See Dosage and Administration (2.1)].
In a study of patients with impaired renal function (mean creatinine clearance of 19 mL/min), the half-life of hydrochlorothiazide elimination was lengthened to 21 hours.
Hepatic Insufficiency : On average, patients with mild-to-moderate chronic liver disease have twice the exposure (measured by AUC values) to valsartan of healthy volunteers (matched by age, sex, and weight). In general, no dosage adjustment is needed in patients with mild-to-moderate liver disease. Care should be exercised in patients with liver disease [See Dosage and Administration (2.1)].
Distribution
Valsartan : The steady state volume of distribution of valsartan after intravenous administration is small (17 L), indicating that valsartan does not distribute into tissues extensively. Valsartan is highly bound to serum proteins (95%), mainly serum albumin.
Hydrochlorothiazide : Hydrochlorothiazide crosses the placental but not the blood-brain barrier and is excreted in breast milk.
Metabolism
Valsartan : The primary metabolite, accounting for about 9% of dose, is valeryl 4-hydroxy valsartan. The enzyme(s) responsible for valsartan metabolism have not been identified but do not seem to be CYP 450 isozymes.
Hydrochlorothiazide : Is not metabolized.
Excretion
Valsartan : Valsartan, when administered as an oral solution, is primarily recovered in feces (about 83% of dose) and urine (about 13% of dose). The recovery is mainly as unchanged drug, with only about 20% of dose recovered as metabolites.
Following intravenous administration, plasma clearance of valsartan is about 2 L/h and its renal clearance is 0.62 L/h (about 30% of total clearance).
Hydrochlorothiazide2 DOSAGE AND ADMINISTRATION2.1 General ConsiderationsThe side effects of valsartan are generally rare and appear independent of dose. Those of hydrochlorothiazide are a mixture of dose-dependent (primarily hypokalemia) and dose-independent phenomena (e.g., pancreatitis), the former much more common than the latter [See Adverse Reactions (6)].
Dose once-daily. Maximum antihypertensive effects are attained within 2 to 4 weeks after a change in dose.
Diovan HCT may be administered with or without food.
Diovan HCT may be administered with other antihypertensive agents.
Elderly patients : No initial dosage adjustment is required for elderly patients.
Renal impairment : The usual regimens of therapy with Diovan HCT may be followed as long as the patient’s creatinine clearance is >30 mL/min. In patients with more severe renal impairment, loop diuretics are preferred to thiazides, so Diovan HCT is not recommended.
Hepatic impairment : Care should be exercised with dosing of Diovan HCT in patients with hepatic impairment. Start with a low dose and titrate slowly in patients with hepatic impairment [S ee Impaired Hepatic Function (5.3)].
2.2 AddOn TherapyA patient whose blood pressure is not adequately controlled with valsartan (or another ARB) alone or hydrochlorothiazide alone may be switched to combination therapy with Diovan HCT.
A patient who experiences dose-limiting adverse reactions on either component alone may be switched to Diovan HCT containing a lower dose of that component in combination with the other to achieve similar blood pressure reductions. The clinical response to Diovan HCT should be subsequently evaluated and if blood pressure remains uncontrolled after 3 to 4 weeks of therapy, the dose may be titrated up to a maximum of 320/25 mg.
2.3 Replacement TherapyDiovan HCT may be substituted for the titrated components.
2.4 Initial TherapyThe usual starting dose is Diovan HCT 160/12.5 mg once daily. The dosage can be increased after 1 to 2 weeks of therapy to a maximum of one 320/25 mg tablet once daily as needed to control blood pressure [See Clinical Studies (14.2)]. Diovan HCT is not recommended as initial therapy in patients with intravascular volume depletion [See Warnings and Precautions (5.2)].
16 HOW SUPPLIED/STORAGE AND HANDLING
Diovan HCT (valsartan and hydrochlorothiazide, USP) is available as non-scored tablets containing valsartan/hydrochlorothiazide 80/12.5 mg, 160/12.5 mg, 160/25 mg, 320/12.5 mg and 320/25 mg. strengths are available as follows.
80/12.5 mg Tablet - Light orange, ovaloid with slightly convex faces debossed CG on one side and HGH on the other side.
Bottles of 90 NDC 0078-0314-34
Unit Dose (blister pack) NDC 0078-0314-06
Box of 100 (strips of 10)
160/12.5 mg Tablet - Dark red, ovaloid with slightly convex faces debossed CG on one side and HHH on the other side.
Bottles of 90 NDC 0078-0315-34
Unit Dose (blister pack) NDC 0078-0315-06
Box of 100 (strips of 10)
Unit Dose (blister Pack of 30) NDC 0078-0315-15
160/25 mg Tablet - Brown orange, ovaloid with slightly convex faces debossed NVR on one side and HXH on the other side.
Bottles of 90 NDC 0078-0383-34
Unit Dose (blister pack) NDC 0078-0383-06
Box of 100 (strips of 10)
Unit Dose (blister pack of 30) NDC 0078-0383-15
320/12.5 mg Tablet - Pink, ovaloid with beveled edge, debossed NVR on one side and HIL on the other side.
Bottles of 90 NDC 0078-0471-34
Unit Dose (blister pack) NDC 0078-0471-06
Box of 100 (strips of 10)
Unit Dose (blister pack of 30) NDC 0078-0471-15
320/25 mg Tablet - Yellow, ovaloid with beveled edge, debossed NVR on one side and CTI on the other side.
Bottles of 90 NDC 0078-0472-34
Unit Dose (blister pack) NDC 0078-0472-06
Box of 100 (strips of 10)
Unit Dose (blister pack of 30) NDC 0078-0472-15
Store at 25ºC (77ºF); excursions permitted to 15-30ºC (59-86ºF) [see USP Controlled Room Temperature].
Protect from moisture.
Dispense in tight container (USP).
PATIENT COUNSELING INFORMATION 17.1 Information for Patients
Pregnancy : Female patients of childbearing age should be told that use of drugs like Diovan HCT that act on the renin-angiotensin system during pregnancy can cause serious problems in the fetus and infant including: low blood pressure, poor development of skull bones, kidney failure and death. Discuss other treatment options with female patients planning to become pregnant. Women using Diovan HCT who become pregnant should notify their physician as soon as possible.
Symptomatic Hypotension : A patient receiving Diovan HCT should be cautioned that lightheadedness can occur, especially during the first days of therapy, and that it should be reported to the prescribing physician. The patients should be told that if syncope occurs, Diovan HCT should be discontinued until the physician has been consulted.
All patients should be cautioned that inadequate fluid intake, excessive perspiration, diarrhea, or vomiting can lead to an excessive fall in blood pressure, with the same consequences of lightheadedness and possible syncope.
Potassium Supplements : A patient receiving Diovan HCT should be told not to use potassium supplements or salt substitutes containing potassium without consulting the prescribing physician.
When pregnancy is detected, discontinue Diovan HCT ® as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus . [ See Warnings and Precautions (5.1) ]
PATIENT INFORMATION
DIOVAN HCT (DYE’ -o -van HCT)
(valsartan and hydrochlorothiazide)
Tablets
Read the Patient Information that comes with DIOVAN HCT before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your condition and treatment. If you have any questions about DIOVAN HCT, ask your doctor or pharmacist.
|
What is the most important
information I should know about DIOVAN HCT?
If you become pregnant, stop taking DIOVAN HCT and call your doctor right away. DIOVAN HCT can harm an unborn baby causing injury and even death. If you plan to become pregnant, talk to your doctor about other treatment options to lower your high blood pressure before taking DIOVAN HCT. |
What is DIOVAN HCT?
DIOVAN HCT contains two prescription medicines:
- valsartan, an angiotensin receptor blocker (ARB)
- hydrochlorothiazide (HCTZ), a water pill (diuretic)
DIOVAN HCT may be used to lower high blood pressure (hypertension) in adults-
- when one medicine to lower your high blood pressure is not enough
- as the first medicine to lower high blood pressure if your doctor decides you are likely to need more than one medicine.
DIOVAN HCT has not been studied in children under 18 years of age.
Who should not take DIOVAN HCT?
Do not take DIOVAN HCT if you:
-
are allergic to any of the ingredients in
DIOVAN HCT. See the end of this leaflet for a complete list of
ingredients in DIOVAN HCT.
- make less urine due to kidney problems
- are allergic to medicines that contain sulfonamides.
What should I tell my doctor before taking DIOVAN HCT?
Tell your doctor about all your medical conditions including if you:
-
are pregnant or plan to become pregnant. See “What
is the most important information I should know about DIOVAN HCT?”
-
are breast
-feeding.
DIOVAN HCT passes into breast milk. You should choose either to take DIOVAN HCT
or breast-feed, but not both.
-
have liver problems
-
have kidney problems
-
have or had gallstones
- have Lupus
Tell your doctor about all the medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements. Some of your other medicines and DIOVAN HCT could affect each other, causing serious side effects. Especially, tell your doctor if you take:
- other medicines for high blood pressure or a heart problem
- water pills (diuretics)
- potassium supplements
- a salt substitute containing potassium
- antidiabetic medicines including insulin
- narcotic pain medicines
- sleeping pills
- lithium, a medicine used in some types of depression (Eskalith®, Lithobid®, Lithium Carbonate, Lithium
Citrate)
- aspirin or other medicines called Non-Steroidal Anti-Inflammatory Drugs (NSAIDs).
Ask your doctor if you are not sure if you are taking one of these medicines.
Know the medicines you take. Keep a list of your medicines with you to show to your doctor and pharmacist when a new medicine is prescribed. Talk to your doctor or pharmacist before you start taking any new medicine. Your doctor or pharmacist will know what medicines are safe to take together.
How should I take DIOVAN HCT?
- Take DIOVAN HCT exactly as prescribed by your doctor. Your doctor may change
your dose if needed.
- Take DIOVAN HCT once each day.
- DIOVAN HCT can be taken with or without food.
- If you miss a dose, take it as soon as you remember. If it is close to your
next dose, do not take the missed dose. Just take the next dose at your regular
time.
- If you take too much DIOVAN HCT, call your doctor or Poison Control Center, or go to the nearest hospital emergency room.
What should I avoid while taking DIOVAN HCT?
You should not take DIOVAN HCT during pregnancy. See “What is the most important information I should know about DIOVAN HCT?”
What are the possible side effects of DIOVAN HCT?
DIOVAN HCT may cause serious side effects including:
-
Harm to an unborn baby causing injury and even death.
See “What is the most important information I should know about DIOVAN
HCT?”
-
Low blood pressure (hypotension). Low blood pressure
is most likely to happen if you:
- take water pills
- are on a low salt diet
- get dialysis treatments
- have heart problems
- get sick with vomiting or diarrhea
- drink alcohol
- take water pills
Lie down if you feel faint or dizzy. Call your doctor right away.
-
Worsening liver problems
.
Liver problems may get worse in people who already have liver problems
and take DIOVAN HCT.
-
Allergic reactions
. People
with and without allergy problems or asthma who take DIOVAN HCT may get allergic
reactions.
-
Worsening of Lupus
.
Hydrochlorothiazide, one of the medicines in DIOVAN HCT may cause Lupus
to become active or worse.
- Fluid and electrolyte (salt) problems . Tell your doctor about any of the following signs and symptoms of fluid and electrolyte problems:
|
|
|
-
K
idney problems. Kidney
problems may become worse in people that already have kidney disease. Some
people will have changes on blood tests for kidney function and may need a lower
dose of DIOVAN HCT. Call your doctor if you get swelling in your feet, ankles,
or hands, or unexplained weight gain. If you have heart failure, your doctor
should check your kidney function before prescribing DIOVAN HCT.
- Skin rash. Call your doctor right away if you have an unusual skin rash.
Other side effects were generally mild and brief. They generally have not caused patients to stop taking DIOVAN HCT.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of DIOVAN HCT. For a complete list, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store DIOVAN HCT?
- Store DIOVAN HCT tablets at room temperature between 59oF to 86oF (15oC
to 30oC).
- Keep DIOVAN HCT in a closed container in a dry place.
Keep DIOVAN HCT and all medicines out of the reach of children.
General information about DIOVAN HCT
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use DIOVAN HCT for a condition for which it was not prescribed. Do not give DIOVAN HCT to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about DIOVAN HCT. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about DIOVAN HCT that is written for health professionals. For more information about DIOVAN HCT, go to www.DIOVAN.com or call 1-866-404-6359.
What are the ingredients in DIOVAN HCT?
Active ingredients: Valsartan and hydrochlorothiazide
Inactive ingredients: colloidal silicon dioxide, crospovidone, hydroxypropyl methylcellulose, iron oxides, magnesium stearate, microcrystalline cellulose, polyethylene glycol, talc, and titanium dioxide.
What is high blood pressure (hypertension)?
Blood pressure is the force in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too much. DIOVAN HCT can help your blood vessels relax and reduce the amount of water in your body so your blood pressure is lower. Medicines that lower blood pressure lower your risk of having a stroke or heart attack.
High blood pressure makes the heart work harder to pump blood throughout the body and causes damage to the blood vessels. If high blood pressure is not treated, it can lead to stroke, heart attack, heart failure, kidney failure, and vision problems.
Eskalith® and Lithobid® are registered trademarks of Noven Pharmaceuticals, Inc.
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
© Novartis
Revised: MAY 2009 T2009-54/T2008-20
DIOVAN LABEL

DIOVAN HCTHYDROCHLOROTHIAZIDE, VALSARTAN TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||