Divalproex Sodium
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- DIVALPROEX SODIUM DESCRIPTION
- INACTIVE INGREDIENT
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- DIVALPROEX SODIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- DIVALPROEX SODIUM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
HEPATOTOXICITYTERATOGENICITY
A PATIENT INFORMATION LEAFLET DESCRIBING THE TERATOGENIC POTENTIAL OF VALPROATE IS AVAILABLE FOR PATIENTS.
PANCREATITIS
WARNINGSPRECAUTIONS
DIVALPROEX SODIUM DESCRIPTION
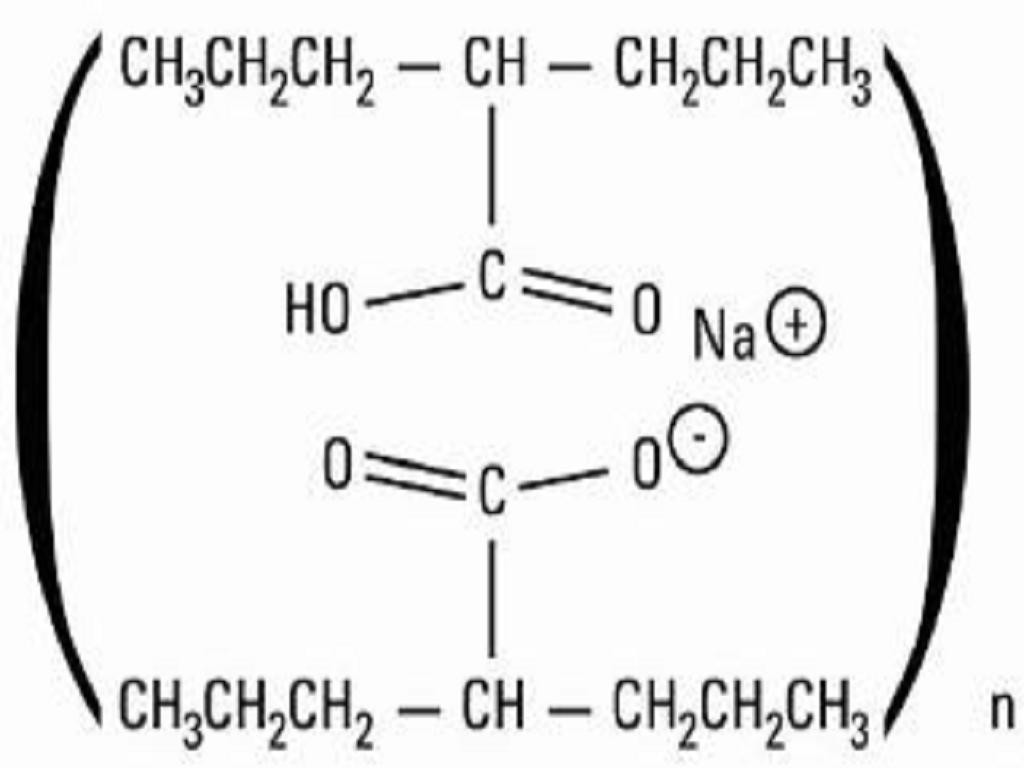
INACTIVE INGREDIENT
CLINICAL PHARMACOLOGY
PharmacodynamicsPharmacokinetics
Absorption/Bioavailability
DOSAGE AND ADMINISTRATION
Distribution
Protein Binding
Drug Interactions
CNS Distribution
Metabolism
Elimination
Special Populations
Effect of Age
DOSAGE AND ADMINISTRATION
Effect of Gender
Effect of Race
Effect of Disease
BOXED WARNINGCONTRAINDICATIONSWARNINGS
Plasma Levels and Clinical Effect
Epilepsy
Mania
DOSAGE AND ADMINISTRATION
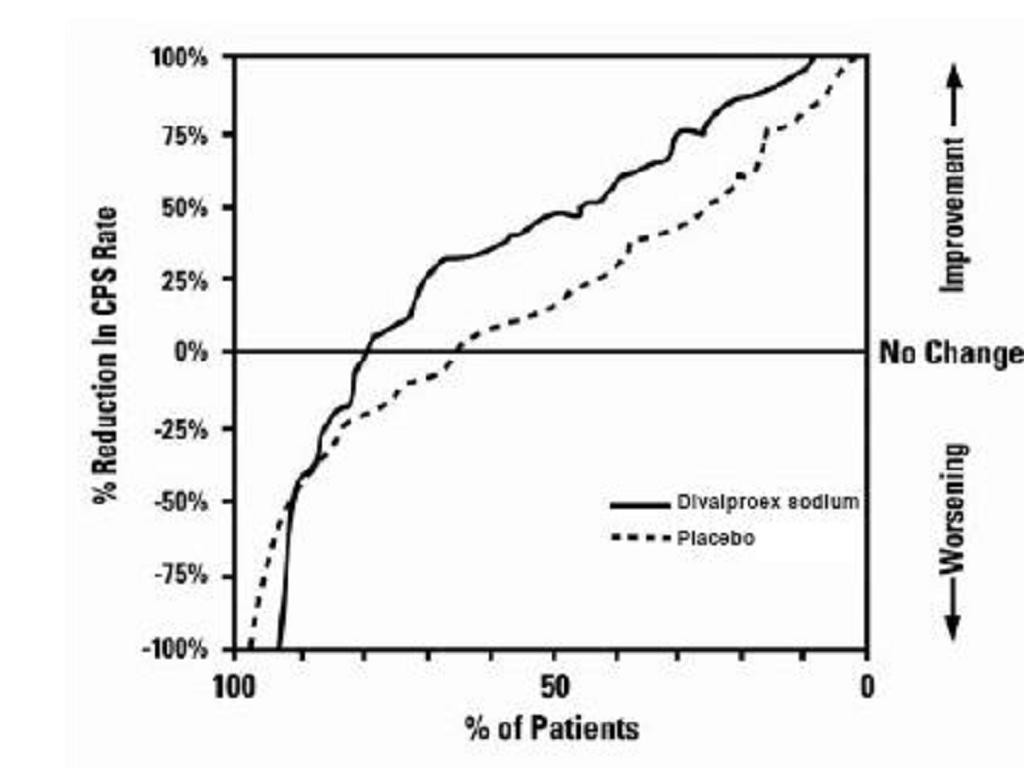
Clinical Trials
Mania
****
*******

Migraine

Epilepsy
**
**

INDICATIONS & USAGE
ManiaClinical Trials
Epilepsy
Migraine
Usage In PregnancyInformation for Patients
WARNINGS
DIVALPROEX SODIUM CONTRAINDICATIONS
WARNINGS
WARNINGS
HepatotoxicityPancreatitis
BOXED WARNING
Urea Cycle Disorders (UCD)
CONTRAINDICATIONSPRECAUTIONS
Usage In Pregnancy
HUMAN DATA
Congenital Malformations
Neural Tube Defects
GENERALWARNINGS
HEPATOTOXICITYBOX WARNING
ANIMAL DATA
Suicidal Behavior and Ideation
Interaction with Carbapenem Antibiotics
Drug Interations
Somnolence in the Elderly
DOSAGE AND ADMINISTRATION
Thrombocytopenia
PRECAUTIONS
PRECAUTIONS
Hepatic DysfunctionBOXED WARNINGCONTRAINDICATIONSWARNINGS
Pancreatitis
BOXED WARNINGWARNINGS
Hypothermia
Drug Interactions
Hyperammonemia
HypothermiaCONTRAINDICATIONSUrea Cycle Disorders(UCD)Hyperammonemia and Encephalopathy Associated with Concomitant Topiramate Use)
CONTRAINDICATIONSUrea Cycle DisordersHyperammonemia
Hyperammonemia and Encephalopathy Associated with Concomitant Topiramate Use
HypothermiaCONTRAINDICATIONSUrea Cycle DisordersHyperammonemia
General
WARNINGS
Drug Interactions
Multi-organ Hypersensitivity Reaction
INFORMATION FOR PATIENTS
Hyperammonemia
Pregnancy
Suicidal Thinking and Behavior
WARNINGS
Multi-organ Hypersensitivity Reaction
Multi-organ Hypersensitivity Reaction
DRUG INTERACTIONS
WARNINGS
CONTRAINDICATIONSUrea CycleDisordersHyperammonemiaHyperammonemia and Encephalopathy Associated with Concomitant TopiramateUseHypothermiaHyperammonemia
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
WARNINGSNURSING MOTHERS
PEDIATRIC USE
BOXED WARNINGGERIATRIC USE
Somnolence in the ElderlyDOSAGE AND ADMINISTRATION
DIVALPROEX SODIUM ADVERSE REACTIONS
Mania*
*
Body as a Whole
Cardiovascular System
Digestive System
Hemic and Lymphatic System
Metabolic and Nutritional Disorders
Musculoskeletal System
Nervous System
Respiratory System
Skin and Appendages
Special Senses
Urogenital System
Migraine
Table 3
*
*
Body as a Whole
Cardiovascular System
Digestive System
Hemic and Lymphatic System
Metabolic and Nutritional Disorders
Musculoskeletal System
Nervous System
Respiratory System
Skin and Appendages
Special Senses
Urogenital System
Epilepsy
*
*
Body as a Whole
Cardiovascular System
Digestive System
Hemic and Lymphatic System
Metabolic and Nutritional Disorders
Musculoskeletal System
Nervous System
Respiratory System
Skin and Appendages
Special Senses
Urogenital System
Other Patient Populations
Gastrointestinal
CNS Effects
WARNINGSPRECAUTIONS
Dermatologic
PRECAUTIONS
Psychiatric
Musculoskeletal
Hematologic
GeneralDrug Interactions
Hepatic
WARNINGS
Endocrine
PRECAUTIONS
Pancreatic
WARNINGS
Metabolic
PRECAUTIONS
Genitourinary
Special Senses
Other
OVERDOSAGE
DOSAGE & ADMINISTRATION
ManiaEpilepsy
PRECAUTIONS
Complex Partial Seizures
Monotherapy (Initial Therapy)
Conversion to Monotherapy
Adjunctive Therapy
CLINICAL STUDIESDrug InteractionsPRECAUTIONS
Simple and Complex Absence Seizures
CLINICAL PHARMACOLOGY
PRECAUTIONS
Migraine
General Dosing Advice
Dosing in Elderly Patients
WARNINGS
Dose-Related Adverse Events
PRECAUTIONS
G.I. Irritation
HOW SUPPLIED
STORAGE AND HANDLING
INFORMATION FOR PATIENTS
Patient Information Leaflet-
● Women taking divalproex sodium delayed-release tablets who are planning to get pregnant should discuss the treatment options with their doctor.
-
● If you become pregnant while taking divalproex sodium delayed-release tablets you should contact your doctor immediately.
-
● Your medication should be taken exactly as prescribed by your doctor to get the most benefit from your medication and reduce the risk of side effects.
-
● If you have taken more than the prescribed dose of your medication, contact your hospital emergency room or local poison center immediately.
-
● Your medication was prescribed for your particular condition. Do not use it for another condition or give the drug to others.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


Divalproex SodiumDivalproex Sodium TABLET, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!