DOCEFREZ
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DOCEFREZ safely and effectively. See full prescribing information for DOCEFREZ. DOCEFREZ (docetaxel) for Injection, Intravenous InfusionInitial U.S. Approval: 1996 RECENT MAJOR CHANGES Dosage and administration (2.9) 07/2012 BOXED WARNING WARNING: TOXIC DEATHS, HEPATOTOXICITY, NEUTROPENIA, HYPERSENSITIVITY REACTIONS, and FLUID RETENTION See full prescribing information for complete boxed warning Treatment-related mortality increases with abnormal liver function, at higher doses, and in patients with NSCLC and prior platinum-based therapy receiving docetaxel at 100 mg/m2 (5.1) Should not be given if bilirubin > ULN, or if AST and/or ALT > 1.5 x ULN concomitant with alkaline phosphatase > 2.5 x ULN. LFT elevations increase risk of severe or life-threatening complications. Obtain LFTs before each treatment cycle (8.6) Should not be given if neutrophil counts are
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: TOXIC DEATHS, HEPATOTOXICITY, NEUTROPENIA, HYPERSENSITIVITY REACTIONS, and FLUID RETENTION
- 1 DOCEFREZ INDICATIONS AND USAGE
- 2 DOCEFREZ DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 DOCEFREZ CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 DOCEFREZ ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 DOCEFREZ DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-DOCEFREZ-20MG-LABEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-DOCEFREZ-20MG-CARTON
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-DILUENT-20MG-LABEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-DOCEFREZ-80MG-LABEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-DOCEFREZ-80MG-CARTON
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-DILUENT-80MG-LABEL
FULL PRESCRIBING INFORMATION
WARNING: TOXIC DEATHS, HEPATOTOXICITY, NEUTROPENIA, HYPERSENSITIVITY REACTIONS, and FLUID RETENTION
The incidence of treatment-related mortality associated with docetaxel therapy is increased in patients with abnormal liver function, in patients receiving higher doses, and in patients with non-small cell lung carcinoma and a history of prior treatment with platinum-based chemotherapy who receive docetaxel as a single agent at a dose of 100 mg/m2 [see Warnings and Precautions (5.1)].
DOCEFREZ should not be given to patients with bilirubin > upper limit of normal (ULN), or to patients with AST and/or ALT >1.5 x ULN concomitant with alkaline phosphatase >2.5 x ULN. Patients with elevations of bilirubin or abnormalities of transaminase concurrent with alkaline phosphatase are at increased risk for the development of grade 4 neutropenia, febrile neutropenia, infections, severe thrombocytopenia, severe stomatitis, severe skin toxicity, and toxic death. Patients with isolated elevations of transaminase >1.5 x ULN also had a higher rate of febrile neutropenia grade 4 but did not have an increased incidence of toxic death. Bilirubin, AST or ALT, and alkaline phosphatase values should be obtained prior to each cycle of DOCEFREZ therapy [see Warnings and Precautions (5.2)].
DOCEFREZ therapy should not be given to patients with neutrophil counts of <1500 cells/mm3. In order to monitor the occurrence of neutropenia, which may be severe and result in infection, frequent blood cell counts should be performed on all patients receiving DOCEFREZ [see Warnings and Precautions (5.3)].
Severe hypersensitivity reactions characterized by generalized rash/erythema, hypotension and/or bronchospasm, or very rarely fatal anaphylaxis, have been reported in patients who received a 3-day dexamethasone premedication. Hypersensitivity reactions require immediate discontinuation of the DOCEFREZ infusion and administration of appropriate therapy [see Warnings and Precautions (5.4)]. DOCEFREZ must not be given to patients who have a history of severe hypersensitivity reactions to docetaxel or to other drugs formulated with polysorbate 80 [see Contraindications (4)].
Severe fluid retention occurred in 6.5% (6/92) of patients despite use of a 3-day dexamethasone premedication regimen. It was characterized by one or more of the following events: poorly tolerated peripheral edema, generalized edema, pleural effusion requiring urgent drainage, dyspnea at rest, cardiac tamponade, or pronounced abdominal distention (due to ascites) [see Warnings and Precautions (5.5)].
1 INDICATIONS AND USAGE
1.1 Breast Cancer
1.2 Non-Small Cell Lung Cancer
1.3 Prostate Cancer
2 DOSAGE AND ADMINISTRATION
[see Dosage and Administration (2.7)].
2.1 Breast Cancer
- For locally advanced or metastatic breast cancer after failure of prior chemotherapy, the recommended dose of DOCEFREZ is 60 mg/m2 to 100 mg/m2 administered intravenously over 1 hour every 3 weeks.
2.2 Non-Small Cell Lung Cancer
- For treatment after failure of prior platinum-based chemotherapy, docetaxel was evaluated as monotherapy, and the recommended dose is 75 mg/m2 administered intravenously over 1 hour every 3 weeks. A dose of 100 mg/m2 in patients previously treated with chemotherapy was associated with increased hematologic toxicity, infection, and treatment-related mortality in randomized, controlled trials [see Boxed Warning, Dosage and Administration (2.7), Warnings and Precautions (5), Clinical Studies (14)].
2.3 Prostate Cancer
- For hormone-refractory metastatic prostate cancer, the recommended dose of DOCEFREZ is 75 mg/m2 every 3 weeks as a 1 hour intravenous infusion. Prednisone 5 mg orally twice daily is administered continuously [see Dosage and Administration (2.7)].
2.6 Premedication Regimen
[see Boxed Warning, Warnings and Precautions (5.4)]. 2
[see Warnings and Precautions (5.4)]
2.7 Dosage Adjustments During Treatment
Breast Cancer
2 3 2 22 2 2 3
Non-Small Cell Lung Cancer
Monotherapy with DOCEFREZ for NSCLC treatment after failure of prior platinum-based chemotherapy
23 2
Prostate Cancer
Combination therapy with DOCEFREZ for hormone-refractory metastatic prostate cancer
33
[see Drug Interactions (7), Clinical Pharmacology (12.3)]
2.8 Administration Precautions
. [see How Supplied/ Storage and Handling (16.3)].
DOCEFREZ (Lyophilized Powder for Injection and Diluent)
| Product | Fill Range of the Diluent (35.4% w/w ethanol in polysorbate 80) | Volume of Diluent to be added for the reconstitution | Concentration of the reconstituted solution |
|---|---|---|---|
| Docetaxel 20 mg vial |
1.10 – 1.15 mL |
1 mL |
20 mg/0.8 mL |
| Docetaxel 80 mg vial |
4.13 – 4.29 mL |
4 mL |
24 mg/mL |
2.9 Preparation and Administration
DOCEFREZ (Lyophilized Powder for Injection and Diluent)
- DOCEFREZ vials should be stored between 2°C and 8°C (36°F and 46°F). Allow the appropriate number of vials of DOCEFREZ (docetaxel) for Injection and diluent (35.4% ethanol in polysorbate 80) vials to stand at room temperature for approximately 5 minutes.
-
a) For DOCEFREZ 20: Use 1 mL syringe with needle of 18- to 21-gauge, 1½-inch for withdrawing diluent for DOCEFREZ 20.
b) For DOCEFREZ 80: Use 4 mL syringe with needle of 18- to 21-gauge, 1½-inch for withdrawing diluent for DOCEFREZ 80. -
a) For DOCEFREZ 20: Aseptically withdraw 1 mL from the diluent vial into a syringe by partially inverting the vial, and transfer it to product vial of DOCEFREZ (docetaxel) for Injection.
b) For DOCEFREZ 80: Aseptically withdraw 4 mL from the diluent vial into a syringe by partially inverting the vial, and transfer it to product vial of DOCEFREZ (docetaxel) for Injection. - Shake the reconstituted vial well in order to completely dissolve the docetaxel powder present in the vial. For the 20 mg vial, the resultant concentration is 20 mg/0.8 mL. For the 80 mg vial, the resultant concentration is 24 mg/mL.
- Aseptically withdraw the required amount of reconstituted DOCEFREZ solution with a calibrated syringe and inject into a 250 mL infusion bag or bottle of either 0.9% Sodium Chloride solution or 5% Dextrose solution to produce a final concentration of 0.3 to 0.74 mg/mL. If a dose greater than 200 mg of DOCEFREZ is required, use a larger volume of the infusion vehicle so that a concentration of 0.74 mg/mL docetaxel is not exceeded.
- Thoroughly mix the infusion by manual rotation.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If the DOCEFREZ reconstituted solution or infusion solution is not clear or appears to have precipitation, these should be discarded.
The final DOCEFREZ infusion solution should be administered intravenously as a 1-hour infusion under ambient room temperature and lighting conditions.
2.10 Stability
3 DOSAGE FORMS AND STRENGTHS
DOCEFREZ (Lyophilized Powder for Injection and Diluent)
DOCEFREZ 80 mg
DOCEFREZ 20 mg
4 CONTRAINDICATIONS
- DOCEFREZ is contraindicated in patients who have a history of severe hypersensitivity reactions to docetaxel or to other drugs formulated with polysorbate 80. Severe reactions, including anaphylaxis, have occurred [see Warnings and Precautions (5.4)].
- DOCEFREZ should not be used in patients with neutrophil counts of <1500 cells/mm3.
5 WARNINGS AND PRECAUTIONS
5.1 Toxic Deaths
Breast Cancer
2 2
Non-Small Cell Lung Cancer
222[see Dosage and Administration (2.2), Clinical Studies (14)].
5.2 Hepatic Impairment
[see Boxed Warning, Use in Specific Populations (8.6), Clinical studies (14)].
5.3 Hematologic Effects
33
3[see Dosage and Administration (2.7)].
3223223
22[see Adverse Reactions (6.1), Clinical Studies (14)].
[see Dosage and Administration (2.7), Adverse Reactions (6)]
5.4 Hypersensitivity Reactions
[see Dosage and Administration (2.6)].
5.5 Fluid Retention
[see Dosage and Administration (2.6)]
22
e.g
5.6 Acute Myeloid Leukemia
5.7 Cutaneous Reactions
[see Dosage and Administration (2.7)]
5.8 Neurologic Reactions
e.g[see Dosage and Administration (2.7)].
5.9 Asthenia
5.10 Use in Pregnancy
[see Use in Specific Populations (8.1)]
6 ADVERSE REACTIONS
- Toxic Deaths [see Boxed Warning, Warnings and Precautions (5.1)]
- Hepatotoxicity [see Boxed Warning, Warnings and Precautions (5.2)]
- Neutropenia [see Boxed Warning, Warnings and Precautions (5.3)]
- Hypersensitivity [see Boxed Warning, Warnings and Precautions (5.4)]
- Fluid Retention [see Boxed Warning, Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Breast Cancer
Monotherapy with docetaxel for locally advanced or metastatic breast cancer after failure of prior chemotherapy
22
| Adverse Reaction | All Tumor Types Normal LFTs n=2045 % |
All Tumor Types Elevated LFTs n=61 % |
Breast Cancer Normal LFTs n=965 % |
|---|---|---|---|
|
Hematologic
|
|||
| Neutropenia |
|||
| <2000 cells/mm3
|
96 |
96 |
99 |
| <500 cells/mm3
|
75 |
88 |
86 |
| Leukopenia |
|||
| <4000 cells/mm3
|
96 |
98 |
99 |
| <1000 cells/mm3
|
32 |
47 |
44 |
| Thrombocytopenia |
|||
| <100,000 cells/mm3
|
8 |
25 |
9 |
| Anemia |
|||
| <11 g/dL |
90 |
92 |
94 |
| <8 g/dL |
9 |
31 |
8 |
Febrile Neutropenia |
11 |
26 |
12 |
|
Septic Death
|
2 |
5 |
1 |
|
Non-Septic Death
|
1 |
7 |
1 |
|
Infections
|
|||
| Any |
22 |
33 |
22 |
| Severe |
6 |
16 |
6 |
|
Fever in Absence of Infection
|
|||
| Any |
31 |
41 |
35 |
| Severe |
2 |
8 |
2 |
|
Hypersensitivity Reactions
|
|||
| Regardless of Premedication |
|||
| Any |
21 |
20 |
18 |
| Severe |
4 |
10 |
3 |
| With 3-day Premedication |
n=92 |
n=3 |
n=92 |
| Any |
15 |
33 |
15 |
| Severe |
2 |
0 |
2 |
|
Fluid Retention
|
|||
| Regardless of Premedication |
|||
| Any |
47 |
39 |
60 |
| Severe |
7 |
8 |
9 |
| With 3-day Premedication |
n=92 |
n=3 |
n=92 |
| Any |
64 |
67 |
64 |
| Severe |
7 |
33 |
7 |
|
Neurosensory
|
|||
| Any |
49 |
34 |
58 |
| Severe |
4 |
0 |
6 |
|
Cutaneous
|
|||
| Any |
48 |
54 |
47 |
| Severe |
5 |
10 |
5 |
|
Nail Changes
|
|||
| Any |
31 |
23 |
41 |
| Severe |
3 |
5 |
4 |
|
Gastrointestinal
|
|||
| Nausea |
39 |
38 |
42 |
| Vomiting |
22 |
23 |
23 |
| Diarrhea |
39 |
33 |
43 |
| Severe |
5 |
5 |
6 |
|
Stomatitis
|
|||
| Any |
42 |
49 |
52 |
| Severe |
6 |
13 |
7 |
|
Alopecia
|
76 |
62 |
74 |
|
Asthenia
|
|||
| Any |
62 |
53 |
66 |
| Severe |
13 |
25 |
15 |
|
Myalgia
|
|||
| Any |
19 |
16 |
21 |
| Severe |
2 |
2 |
2 |
|
Arthralgia
|
9 |
7 |
8 |
|
Infusion Site Reactions
|
4 |
3 |
4 |
[see Warnings and Precautions (5.3)]. 3
3
3
Hypersensitivity Reactions
[see Boxed Warning, Warnings and Precautions (5.4)]
Fluid Retention
[see Boxed Warning, Dosage and Administration (2.6), Warnings and Precautions (5.5)]
Cutaneous Reactions
[see Warnings and Precautions (5.7)].
Neurologic Reactions
[ see Warnings and Precautions (5.8)].
Gastrointestinal Reactions
Cardiovascular Reactions
2
Infusion Site Reactions
Hepatic Reactions
Hematologic and Other Toxicity: Relation to dose and baseline liver chemistry abnormalities
22
| Adverse Reaction | Docetaxel 100 mg/m2 | Docetaxel 60 mg/m2 | |
|---|---|---|---|
Normal LFTs n=730 % |
Elevated LFTs n=18 % |
Normal LFTs n=174 % |
|
|
Neutropenia
|
|||
| Any <2000 cells/mm3
|
98 |
100 |
95 |
| Grade 4 <500 cells/mm3
|
84 |
94 |
75 |
|
Thrombocytopenia
|
|||
| Any <100,000 cells/mm3
|
11 |
44 |
14 |
| Grade 4 <20,000 cells/mm3
|
1 |
17 |
1 |
|
Anemia <11 g/dL |
95 |
94 |
65 |
Infection
 |
|||
| Any |
23 |
39 |
1 |
| Grade 3 and 4 |
7 |
33 |
0 |
Febrile Neutropenia
 |
|||
| By Patient |
12 |
33 |
0 |
| By Course |
2 |
9 |
0 |
|
Septic Death
|
2 |
6 |
1 |
|
Non-Septic Death
|
1 |
11 |
0 |
| Adverse Reaction | Docetaxel 100 mg/m2 | Docetaxel 60 mg/m2 | |
|---|---|---|---|
Normal LFTs n=730 % |
Elevated LFTs n=18 % |
Normal LFTs n=174 % |
|
| NA = not available |
|||
|
Acute Hypersensitivity Reaction
|
|||
|
Regardless of Premedication
|
|||
| Any |
13 |
6 |
1 |
| Severe |
1 |
0 |
0 |
Fluid Retention |
|||
|
Regardless of Premedication
|
|||
| Any |
56 |
61 |
13 |
| Severe |
8 |
17 |
0 |
|
Neurosensory
|
|||
| Any |
57 |
50 |
20 |
| Severe |
6 |
0 |
0 |
|
Myalgia
|
23 |
33 |
3 |
|
Cutaneous
|
|||
| Any |
45 |
61 |
31 |
| Severe |
5 |
17 |
0 |
|
Asthenia
|
|||
| Any |
65 |
44 |
66 |
| Severe |
17 |
22 |
0 |
|
Diarrhea
|
|||
| Any |
42 |
28 |
NA |
| Severe |
6 |
11 |
|
|
Stomatitis
|
|||
| Any |
53 |
67 |
19 |
| Severe |
8 |
39 |
1 |
222
Lung Cancer
Monotherapy with docetaxel for unresectable, locally advanced or metastatic NSCLC previously treated with platinum-based chemotherapy
2
| Adverse Reaction | Docetaxel 75 mg/m2
n=176 % |
Best Supportive Care n=49 % |
Vinorelbine/Ifosfamide n=119 % |
|---|---|---|---|
|
Neutropenia
|
|||
| Any |
84 |
14 |
83 |
| Grade 3/4 |
65 |
12 |
57 |
|
Leukopenia
|
|||
| Any |
84 |
6 |
89 |
| Grade 3/4 |
49 |
0 |
43 |
|
Thrombocytopenia
|
|||
| Any |
8 |
0 |
8 |
| Grade 3/4 |
3 |
0 |
2 |
|
Anemia
|
|||
| Any |
91 |
55 |
91 |
| Grade 3/4 |
9 |
12 |
14 |
Febrile Neutropenia |
6 |
NA |
1 |
|
Infection
|
|||
| Any |
34 |
29 |
30 |
| Grade 3/4 |
10 |
6 |
9 |
|
Treatment Related Mortality
|
3 |
NA |
3 |
|
Hypersensitivity Reactions
|
|||
| Any |
6 |
0 |
1 |
| Grade 3/4 |
3 |
0 |
0 |
|
Fluid Retention
|
|||
| Any |
34 |
ND |
23 |
| Severe |
3 |
3 |
|
|
Neurosensory
|
|||
| Any |
23 |
14 |
29 |
| Grade 3/4 |
2 |
6 |
5 |
|
Neuromotor
|
|||
| Any |
16 |
8 |
10 |
| Grade 3/4 |
5 |
6 |
3 |
|
Skin
|
|||
| Any |
20 |
6 |
17 |
| Grade 3/4 |
1 |
2 |
1 |
|
Gastrointestinal
|
|||
| Nausea |
|||
| Any |
34 |
31 |
31 |
| Grade 3/4 |
5 |
4 |
8 |
| Vomiting |
|||
| Any |
22 |
27 |
22 |
| Grade 3/4 |
3 |
2 |
6 |
| Diarrhea |
|||
| Any |
23 |
6 |
12 |
| Grade 3/4 |
3 |
0 |
4 |
|
Alopecia
|
56 |
35 |
50 |
|
Asthenia
|
|||
| Any |
53 |
57 |
54 |
Severe |
18 |
39 |
23 |
|
Stomatitis
|
|||
| Any |
26 |
6 |
8 |
| Grade 3/4 |
2 |
0 |
1 |
|
Pulmonary
|
|||
| Any |
41 |
49 |
45 |
| Grade 3/4 |
21 |
29 |
19 |
|
Nail Disorder
|
|||
| Any |
11 |
0 |
2 |
Severe |
1 |
0 |
0 |
|
Myalgia
|
|||
| Any |
6 |
0 |
3 |
Severe |
0 |
0 |
0 |
|
Arthralgia
|
|||
| Any |
3 |
2 |
2 |
Severe |
0 |
0 |
1 |
|
Taste Perversion
|
|||
| Any |
6 |
0 |
0 |
Severe |
1 |
0 |
0 |
Combination therapy with docetaxel in patients with prostate cancer
| Docetaxel 75 mg/m2 every 3 weeks + prednisone 5 mg twice daily n=332 % |
Mitoxantrone 12 mg/m2 every 3 weeks + prednisone 5 mg twice daily n=335 % |
|||
|---|---|---|---|---|
| Adverse Reaction | Any | Grade 3/4 | Any | Grade 3/4 |
| Anemia |
67 |
5 |
58 |
2 |
| Neutropenia |
41 |
32 |
48 |
22 |
| Thrombocytopenia |
3 |
1 |
8 |
1 |
| Febrile neutropenia |
3 |
N/A |
2 |
N/A |
| Infection |
32 |
6 |
20 |
4 |
| Epistaxis |
6 |
0 |
2 |
0 |
| Allergic Reactions |
8 |
1 |
1 |
0 |
Fluid Retention Weight Gain  Peripheral Edema  |
24 8 18 |
1 0 0 |
5 3 2 |
0 0 0 |
| Neuropathy Sensory |
30 |
2 |
7 |
0 |
| Neuropathy Motor |
7 |
2 |
3 |
1 |
| Rash/Desquamation |
6 |
0 |
3 |
1 |
| Alopecia |
65 |
N/A |
13 |
N/A |
| Nail Changes |
30 |
0 |
8 |
0 |
| Nausea |
41 |
3 |
36 |
2 |
| Diarrhea |
32 |
2 |
10 |
1 |
| Stomatitis/Pharyngitis |
20 |
1 |
8 |
0 |
| Taste Disturbance |
18 |
0 |
7 |
0 |
| Vomiting |
17 |
2 |
14 |
2 |
| Anorexia |
17 |
1 |
14 |
0 |
| Cough |
12 |
0 |
8 |
0 |
| Dyspnea |
15 |
3 |
9 |
1 |
| Cardiac left ventricular function |
10 |
0 |
22 |
1 |
| Fatigue |
53 |
5 |
35 |
5 |
| Myalgia |
15 |
0 |
13 |
1 |
| Tearing |
10 |
1 |
2 |
0 |
| Arthralgia |
8 |
1 |
5 |
1 |
6.2 Post-marketing Experiences
Body as a whole
Cardiovascular
Cutaneous
Gastrointestinal
Hematologic
Hypersensitivity
Hepatic
Neurologic
Ophthalmologic
Hearing
Respiratory
Renal
7 DRUG INTERACTIONS
In vitro
In vivo[see Dosage and Administration (2.7) and Clinical Pharmacology (12.3)]
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
[see ‘Warnings and Precautions’ section]
2
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
Prostate Cancer
8.6 Hepatic Impairment
[see Boxed Warning, Warnings and Precautions (5.2), Clinical Pharmacology (12.3)].
10 OVERDOSAGE
22
22222
11 DESCRIPTION
tert
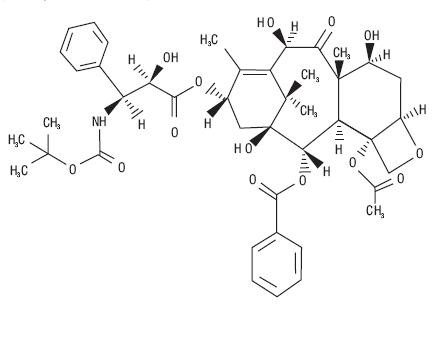 435314
435314DOCEFREZ (Lyophilized Powder for Injection and Diluent)
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
Absorption:22222
Distribution:In vitro1in vitro
Metabolism:In vitro[see Drug Interactions (7)]
Elimination:14tert
Effect of Age:2
Effect of Gender:
Hepatic Impairment:[see Warnings and Precautions (5.2) and Use in Specific Populations (8.6)].
Effect of Race:222
Effect of Ketoconazole[see Dosage and Administration (2.7) and Drug-Drug Interactions (7)]
Effect of Combination Therapies:
Dexamethasone
Prednisone
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
in vitro1in vivothth2
th2rdth2
14 CLINICAL STUDIES
14.1 Locally Advanced or Metastatic Breast Cancer
Randomized Trials
222
|
Efficacy Parameter
|
Docetaxel
(n=203) |
Mitomycin/
Vinblastine (n=189) |
p-value
|
| Median Survival |
11.4 months |
8.7 months |
p=0.01 Log Rank |
Risk Ratio 95% CI (Risk Ratio) |
0.73 0.58-0.93 |
||
| Median Time to Progression |
4.3 months |
2.5 months |
p=0.01 Log Rank |
Risk Ratio 95% CI (Risk Ratio) |
0.75 0.61-0.94 |
||
| Overall Response Rate Complete Response Rate |
28.1% 3.4% |
9.5% 1.6% |
p<0.0001 Chi Square |
| Efficacy Parameter | Docetaxel (n=161) | Doxorubicin (n=165) | p-value |
|---|---|---|---|
| Median Survival |
14.7 months |
14.3 months |
p=0.39 Log Rank |
Risk Ratio 95% CI (Risk Ratio) |
0.89 0.68-1.16 |
||
| Median Time to Progression |
6.5 months |
5.3 months |
p=0.45 Log Rank |
Risk Ratio 95% CI (Risk Ratio) |
0.93 0.71-1.16 |
||
| Overall Response Rate Complete Response Rate |
45.3% 6.8% |
29.7% 4.2% |
p=0.004 Chi Square |
Single Arm Studies
2
22
14.3 Non-Small Cell Lung Cancer (NSCLC)
Monotherapy with Docetaxel for NSCLC Previously Treated with Platinum-Based Chemotherapy
22 Boxed Warning, Dosage and Administration (2.7), Warnings and Precautions (5.3)]
222
22222
| TAX317 | TAX320 | |||
|---|---|---|---|---|
| Docetaxel 75 mg/m2 n=55 |
Best Supportive Care n=49 |
Docetaxel 75 mg/m2 n=125 |
Control (V/I  n=123 |
|
| Overall Survival Log-rank Test |
p=0.01 |
p=0.13 |
||
Risk Ratio 95% CI (Risk Ratio) |
0.56 (0.35, 0.88) |
0.82 (0.63, 1.06) |
||
| Median Survival 95% CI |
7.5 months (5.5, 12.8) |
4.6 months (3.7, 6.1) |
5.7 months (5.1, 7.1) |
5.6 months (4.4, 7.9) |
| % 1-year Survival 95% CI |
37%  (24, 50) |
12% (2, 23) |
30%  (22, 39) |
20% (13, 27) |
| Time to Progression 95% CI |
12.3 weeks (9.0, 18.3) |
7.0 weeks (6.0, 9.3) |
8.3 weeks (7.0, 11.7) |
7.6 weeks (6.7, 10.1) |
| Response Rate 95% CI |
5.5% (1.1, 15.1) |
Not Applicable |
5.7% (2.3, 11.3) |
0.8% (0.0, 4.5) |
Figure 1- TAX317 Survival K-M Curves - Docetaxel 75 mg/m2 vs. Best Supportive Care

Figure 2 - TAX320 Survival K-M Curves – Docetaxel 75 mg/m2 vs. Vinorelbine or Ifosfamide Control

2
14.4 Hormone Refractory Prostate Cancer
- Docetaxel 75 mg/m2 every 3 weeks for 10 cycles.
- Docetaxel 30 mg/m2 administered weekly for the first 5 weeks in a 6-week cycle for 5 cycles.
- Mitoxantrone 12 mg/m2 every 3 weeks for 10 cycles.
| Docetaxel+Prednisone every 3 weeks |
Mitoxantrone+Prednisone every 3 weeks |
|
|---|---|---|
| Number of patients Median survival (months) 95% CI Hazard ratio 95% CI p-value  |
335 18.9 (17.0-21.2) 0.761 (0.619-0.936) 0.0094 |
337 16.5 (14.4-18.6) -- -- -- |

15 REFERENCES
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- American Society of Health-System Pharmacists. (2006) ASHP Guidelines on Handling Hazardous Drugs. Am J Health-Syst Pharm. 2006;63:1172-1193
- Polovich, M., White, J. M., & Kelleher, L.O. (eds.) 2005. Chemotherapy and biotherapy guidelines and recommendations for practice (2nd. ed.) Pittsburgh , PA : Oncology Nursing Society.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
DOCEFREZ (Lyophilized Powder for Injection and Diluent)
16.2 Storage
16.3 Handling and Disposal
[see References (15)]
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling
- DOCEFREZ may cause fetal harm. Advise patients to avoid becoming pregnant while receiving this drug. Women of childbearing potential should use effective contraceptives if receiving DOCEFREZ [see Warnings and Precautions (5.10) and Use in Specific Populations (8.1)].
- Obtain detailed allergy and concomitant drug information from the patient prior to DOCEFREZ administration.
- Explain the significance of oral corticosteroids such as dexamethasone administration to the patient to help facilitate compliance. Instruct patients to report if they were not compliant with oral corticosteroid regimen.
- Instruct patients to immediately report signs of a hypersensitivity reaction.
- Tell patients to watch for signs of fluid retention such as peripheral edema in the lower extremities, weight gain and dyspnea.
- Explain the significance of routine blood cell counts. Instruct patients to monitor their temperature frequently and immediately report any occurrence of fever.
- Instruct patients to report myalgia, cutaneous, or neurologic reactions.
- Explain to patients that side effects such as nausea, vomiting, diarrhea, constipation, fatigue, excessive tearing, infusion site reactions, and hair loss are associated with docetaxel administration.
Patient Information
DOCEFREZ™ (‘dō-sə-‘frāz)
(docetaxel)
for Injection
What is the most important information I should know about DOCEFREZ?
DOCEFREZ can cause serious side effects, including death.
-
The chance of death in people who receive DOCEFREZ is higher if you:
- have liver problems
- receive high doses of DOCEFREZ
- have non-small cell lung cancer and have been treated with chemotherapy medicines that contain platinum
- DOCEFREZ can affect your blood cells. Your doctor should do routine blood tests during treatment with DOCEFREZ. This will include regular checks of your white blood cell counts. If your white blood cells are too low, your doctor may not treat you with DOCEFREZ until you have enough white blood cells. People with low white blood counts can develop life-threatening infections. The earliest sign of infection may be fever. Follow your doctor’s instructions for how often to take your temperature while taking DOCEFREZ. Call your doctor right away if you have a fever.
-
Serious allergic reactions can happen in people who take DOCEFREZ. Serious allergic reactions are medical emergencies that can lead to death and must be treated right away. Tell your doctor right away if you have any of these signs of a serious allergic reaction:
- trouble breathing
- sudden swelling of your face, lips, tongue, throat, or trouble swallowing
- hives (raised bumps), rash, or redness all over your body
- Your body may hold too much fluid (severe fluid retention) during treatment with DOCEFREZ. This can be life threatening. To decrease the chance of this happening, you must take another medicine, a corticosteroid, before each DOCEFREZ treatment. You must take the corticosteroid exactly as your doctor tells you. Tell your doctor or nurse before your DOCEFREZ treatment if you forget to take corticosteroid dose or do not take it as your doctor tells you.
DOCEFREZ
- breast cancer
- non-small cell lung cancer
- prostate cancer
Who should not take DOCEFREZ?
- have had a severe allergic reaction to:
- docetaxel, the active ingredient in DOCEFREZ, or
- any other medicines that contain polysorbate 80. Ask your doctor or pharmacist if you are not sure.
See “What is the most important information I should know about DOCEFREZ?” for the signs and symptoms of a severe allergic reaction.
- have a low white blood cell count.
- are allergic to any medicines. See “Who should not take DOCEFREZ?” Also, see the end of this leaflet for a list of the ingredients in DOCEFREZ.
- have liver problems
- have any other medical conditions
- are pregnant or plan to become pregnant. DOCEFREZ can harm your unborn baby.
- are breast-feeding or plan to breast-feed. It is not known if DOCEFREZ passes into your breast milk. You and your doctor should decide if you will take DOCEFREZ or breast-feed.
How will I receive DOCEFREZ?
- DOCEFREZ will be given to you as an intravenous injection into your vein, usually over 1 hour.
- DOCEFREZ is usually given every 3 weeks.
- Your doctor will decide how long you will receive treatment with DOCEFREZ
- Your doctor will check your blood cell counts and other blood tests during your treatment with DOCEFREZ to check for side effects of DOCEFREZ.
- Your doctor may stop your treatment, change the timing of your treatment, or change the dose of your treatment if you have certain side effects while taking DOCEFREZ.
DOCEFREZ may cause serious side effects including death.
- See “What is the most important information I should know about DOCEFREZ?”
- Acute Myeloid Leukemia (AML), a type of blood cancer, can happen in people who take DOCEFREZ along with certain other medicines. Tell your doctor about all the medicines you take.
- Other Blood Disorders – Changes in blood counts due to leukemia and other blood disorders may occur years after treatment with Docefrez.
- Skin Reactions including redness and swelling of your arms and legs with peeling of your skin.
- Neurologic Symptoms including numbness, tingling, or burning in your hands and feet.
- changes in your sense of taste
- feeling short of breath
- constipation
- decreased appetite
- changes in your fingernails or toenails
- swelling of your hands, face or feet
- feeling weak or tired
- joint and muscle pain
- nausea and vomiting
- diarrhea
- mouth or lips sores
- hair loss
- rash
- redness of the eye, excess tearing
- skin reactions at the site of DOCEFREZ administration such as increased skin pigmentation, redness, tenderness, swelling, warmth or dryness of the skin.
- tissue damage if DOCEFREZ leaks out of the vein into the tissues
General information about DOCEFREZ
What are the ingredients in DOCEFREZ?
|
Every three-week injection of DOCEFREZ for breast, and non-small cell lung cancers
Take your oral corticosteroid medicine as your doctor tells you. Oral corticosteroid dosing: Day 1 Date:_________ Time:______AM _______PM Day 2 Date:_________ Time:______AM _______PM (DOCEFREZ Treatment Day) Day 3 Date:_________ Time:______AM _______PM |
|
Every three-week injection of DOCEFREZ for prostate cancer Take your oral corticosteroid medicine as your doctor tells you. Oral corticosteroid dosing: Date:___________ Time:___________ Date:___________ Time:___________ (DOCEFREZ Treatment Day) Time:___________ |
Sun Pharmaceutical Ind. Ltd.
Caraco Pharmaceutical Laboratories, Ltd.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-DOCEFREZ-20MG-LABEL
NDC 47335-285-40
DocefrezTM
(Docetaxel) for Injection
20 mg
For Intravenous Infusion Only
Each Docefrez for Injection vial contains a slight overfill to deliver 20 mg of Docetaxel per 0.8 mL after reconstitution
Rx ONLY

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-DOCEFREZ-20MG-CARTON
NDC 47335-285-41
DocefrezTM
(Docetaxel) for Injection
20 mg*
For Intravenous Infusion Only
*Each Docefrez for Injection vial contains a slight overfill to deliver 20 mg of Docetaxel per 0.8 mL after reconstitution
Each carton contains:
One vial of Docefrez (docetaxel) for Injection 20 mg
One vial of DILUENT for Docefrez 20 mg
Rx ONLY

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-DILUENT-20MG-LABEL
NDC 47335-287-40
DILUENT for DocefrezTM 20 mg
1.13 mL
Single Use Vial-Discard Unused Portion.
Rx ONLY

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-DOCEFREZ-80MG-LABEL
NDC 47335-286-40
DocefrezTM
(Docetaxel) for Injection
80 mg
For Intravenous Infusion Only
Each Docefrez for Injection vial contains a slight overfill to deliver 80 mg of Docetaxel
(24 mg/mL after reconstitution)

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-DOCEFREZ-80MG-CARTON
NDC 47335-286-41
DocefrezTM
(Docetaxel) for Injection
80 mg*
For Intravenous Infusion Only
*Each Docefrez for Injection vial contains a slight overfill to deliver 80 mg of Docetaxel (24 mg/mL after reconstitution)
Each carton contains:
One vial of Docefrez (docetaxel) for Injection 80 mg
One vial of DILUENT for Docefrez 80 mg
Rx ONLY

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-DILUENT-80MG-LABEL
NDC 47335-288-40
DILUENT for DocefrezTM 80 mg
4.21 mL
Rx ONLY

DOCEFREZdocetaxel anhydrous KIT
| ||||||||||||||||||||||||||||||||||||||||
DOCEFREZdocetaxel anhydrous KIT
| ||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!