Dolce and Gabbana The Lift Foundation Almond 150
Dolce and Gabbana The Lift Foundation Almond 150
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Product Label
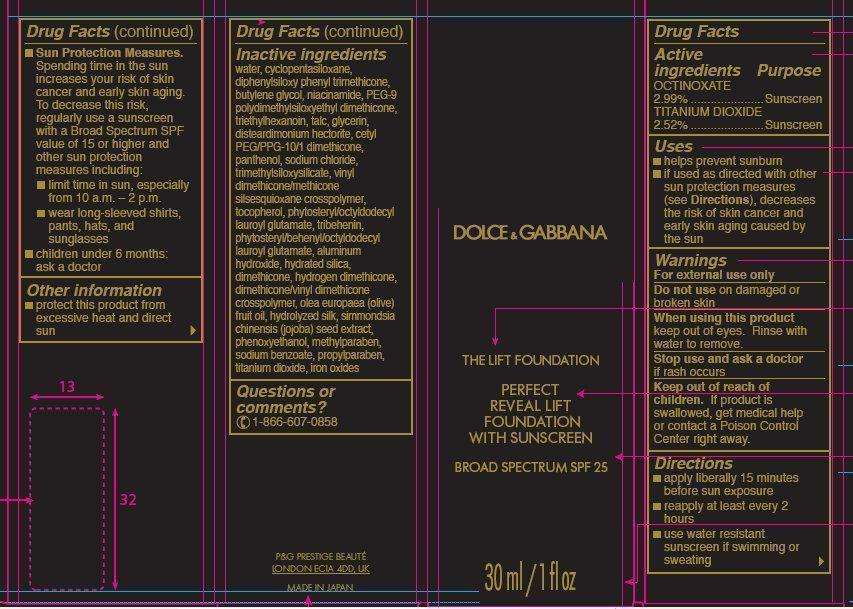


Dolce and Gabbana The Lift Foundation Almond 150OCTINOXATE, TITANIUM DIOXIDE LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!