Dr. Cocoa Nighttime Cough and Cold
Infirst Healthcare
Woodfield Pharmaceutical, LLC
Dr. Cocoa Nighttime Cough+Cold
FULL PRESCRIBING INFORMATION: CONTENTS*
- Drug Facts
- Dr. Cocoa Nighttime Cough and Cold Uses
- Warnings
- Dr. Cocoa Nighttime Cough and Cold Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 120 mL Bottle Label
- PRINCIPAL DISPLAY PANEL - 120 mL Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredients
(in each 5 mL)
Diphenhydramine HCl 6.25 mg
Phenylephrine HCl 2.5 mg
Purpose
Antihistamine/Cough suppressant
Nasal decongestant
Dr. Cocoa Nighttime Cough and Cold Uses
- temporarily relieves:
- sneezing
- runny nose
- itchy nose or throat
- itchy, watery eyes
- cough due to minor throat and bronchial irritation as may occur with a cold
- nasal congestion
Warnings
Do not use
- to make a child sleepy
- in a child under 6 years of age
- with any other drug containing diphenhydramine, even one used on the skin
- in a child now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before using this product.
Ask a doctor before use if your child has
- heart disease
- diabetes
- high blood pressure
- thyroid disease
- glaucoma
- trouble urinating due to an enlarged prostate gland
- breathing problems such chronic bronchitis
- persistent or chronic cough such as occurs with asthma
- cough that occurs with too much phlegm (mucus)
Ask a doctor or pharmacist before use if your child is taking sedatives or tranquilizers
When using this product
- do not use more than directed
- excitability in your child may occur
- marked drowsiness in your child may occur
- sedatives and tranquilizers may increase drowsiness
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- symptoms do not improve within 7 days or occur with a fever
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or headache that lasts. These could be signs of a serious condition.
Keep out of reach of children. Dr. CocoaTM is a medicine. In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
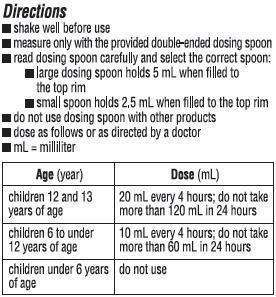
Dr. Cocoa Nighttime Cough and Cold Other information
- each 5 mL contains: sodium 7 mg
- store at 20 - 25°C (68 -77°F)
Inactive ingredients
anhydrous citric acid, cocoa, maltitol, methylparaben, natural flavor, propylene glycol, purified water, sodium benzoate, sorbitol, sucralose, trisodium citrate dihydrate
Questions or comments?
call 1-855-848-3284 toll free
PRINCIPAL DISPLAY PANEL - 120 mL Bottle Label
Dr.
Cocoa™
....................
for Children
Nighttime
Cough+Cold
Diphenhydramine HCl
(Antihistamine/Cough Suppressant)
Phenylephrine HCl (Nasal Decongestant)
For Ages
6-13
4 FL OZ (120 mL)

PRINCIPAL DISPLAY PANEL - 120 mL Carton
NDC 62372-743-04
NEW!
Dr.
Cocoa™
....................
for Children
Nighttime
Cough+Cold
Diphenhydramine HCl
(Antihistamine/Cough Suppressant)
Phenylephrine HCl (Nasal Decongestant)
Relieves:
Cough
Stuffy Nose
Runny Nose
Sneezing
Helps Your
Child Rest
For Ages 6-13
Real Chocolate Taste
4 FL OZ (120 mL)

Dr. Cocoa Nighttime Cough and ColdDiphenhydramine Hydrchloride, Phenylephrine Hydrochloride LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||