Dysport
Ipsen Biopharmaceuticals, Inc.
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DYSPORT for Injection safely and effectively. See full prescribing information for DYSPORT for Injection. DYSPORT for Injection(abobotulinumtoxinA)Initial U.S. Approval: April 2009BOXED WARNINGDistant Spread of Toxin EffectThe effects of DYSPORT ® and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults, particularly in those patients who have underlying conditions that would predispose them to these symptoms.INDICATIONS AND USAGEDYSPORT ® is an acetylcholine release inhibitor and a neuromuscular blocking agent indicated for: the treatment of adults with cervical dystonia to reduce the severity of abnormal head position and neck pain in both toxin-naïve and previously treated patients (1.1) the temporary improvement in the appearance of moderate to severe glabellar lines associated with procerus and corrugator muscle activity in adult patients < 65 years of age (1.2) DOSAGE AND ADMINISTRATION Once reconstituted, DYSPORT ® should be stored in the original container in a refrigerator (2–8°C) and used within 24 hours (16) Do not freeze after reconstitution (2), (16) Protect from light (16) Reconstitution instructions are specific for the 300 Unit and 500 Unit vials Cervical Dystonia (2.1) Initial dose of DYSPORT ® is 500 Units given intramuscularly as a divided dose among the affected muscles Re-treatment every 12 to 16 weeks or longer, as necessary, based on return of clinical symptoms with doses administered between 250 and 1000 Units to optimize clinical benefit Re-treatment should not occur in intervals of less than 12 weeks Titration should occur in 250 Unit steps according to the patient's response Glabellar Lines (2.2) A total dose of 50 Units of DYSPORT ® , divided in five equal aliquots of 10 Units each, should be administered to affected muscles to achieve clinical effect Re-treatment with DYSPORT ® should be administered no more frequently than every 3 months DOSAGE FORMS AND STRENGTHS Cervical dystonia: Single-use, sterile 500 Unit vial for reconstitution with 1 mL of 0.9 % Sodium Chloride Injection USP (without preservative) and a single use, sterile 300 Unit vial for reconstitution with 0.6 mL of 0.9% Sodium Chloride Injection USP (without preservative) (3.1) Glabellar lines: Single-use, sterile 300 Unit vial for reconstitution with 2.5 mL or 1.5 mL of 0.9% Sodium Chloride Injection USP (without preservative) (3.2) CONTRAINDICATIONS Hypersensitivity to any botulinum toxin product or excipients (4), (6.1), (6.2) Allergy to cow's milk protein (4) Infection at the proposed injection site(s) (4) WARNINGS AND PRECAUTIONS The potency Units of DYSPORT® are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of DYSPORT® cannot be compared to or converted into units of any other botulinum toxin products (11) Recommended dose and frequency of administration should not be exceeded (5.1) Immediate medical attention may be required in cases of respiratory, speech or swallowing difficulties (5.3) Caution should be exercised when administering DYSPORT ® to patients with surgical alterations to the facial anatomy, marked facial asymmetry, inflammation at the injection site(s), ptosis, excessive dermatochalasis, deep dermal scarring, or thick sebaceous skin (5.4) Concomitant neuromuscular disorder may exacerbate clinical effects of treatment (5.5) DYSPORT ® contains human albumin. Based on effective donor screening and product manufacturing processes, DYSPORT ® carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) also is considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for albumin (5.6) The possibility of an immune reaction when injected intradermally is unknown. The safety of DYSPORT ® for the treatment of hyperhidrosis has not been established (5.7) Side Effects Cervical Dystonia Most commonly observed adverse reactions (> 5% of patients) are: muscular weakness, dysphagia, dry mouth, injection site discomfort, fatigue, headache, neck pain, musculoskeletal pain, dysphonia, injection site pain, and eye disorders. (6) Glabellar Lines The most frequently reported adverse events (≥2%) are nasopharyngitis, headache, injection site pain, injection site reaction, upper respiratory tract infection, eyelid edema, eyelid ptosis, sinusitis and nausea. (6) To report SUSPECTED ADVERSE REACTIONS, contact 877-397-7671 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONS Patients receiving concomitant treatment of DYSPORT ® and aminoglycosides or other agents interfering with neuromuscular transmission (e.g., curare-like agents), or muscle relaxants, should be observed closely because the effect of botulinum toxin may be potentiated (7) Use of anticholinergic drugs may potentiate systemic anticholinergic effects (7) The effect of administering different botulinum neurotoxins during the course of treatment with DYSPORT ® is unknown (7) USE IN SPECIFIC POPULATIONS Pregnancy: Based on animal data, may cause fetal harm (8.1) Care should be exercised when administering DYSPORT ® in elderly patients, reflecting the greater frequency of concomitant disease and other drug therapy (8.5)
FULL PRESCRIBING INFORMATION: CONTENTS*
- Distant Spread of Toxin Effect
- 1 DYSPORT INDICATIONS AND USAGE
- 2 DYSPORT DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 DYSPORT CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 5.1 Lack of Interchangeability between Botulinum Toxin Products
- 5.2 Spread of Toxin Effect
- 5.3 Dysphagia and Breathing Difficulties in Treatment of Cervical Dystonia
- 5.4 Facial Anatomy in the Treatment of Glabellar Lines
- 5.5 Pre-existing Neuromuscular Disorders
- 5.6 Human Albumin
- 5.7 Intradermal Immune Reaction
- 6 DYSPORT ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 DYSPORT DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PRINCIPAL DISPLAY PANEL - Vial Carton - 500 Units
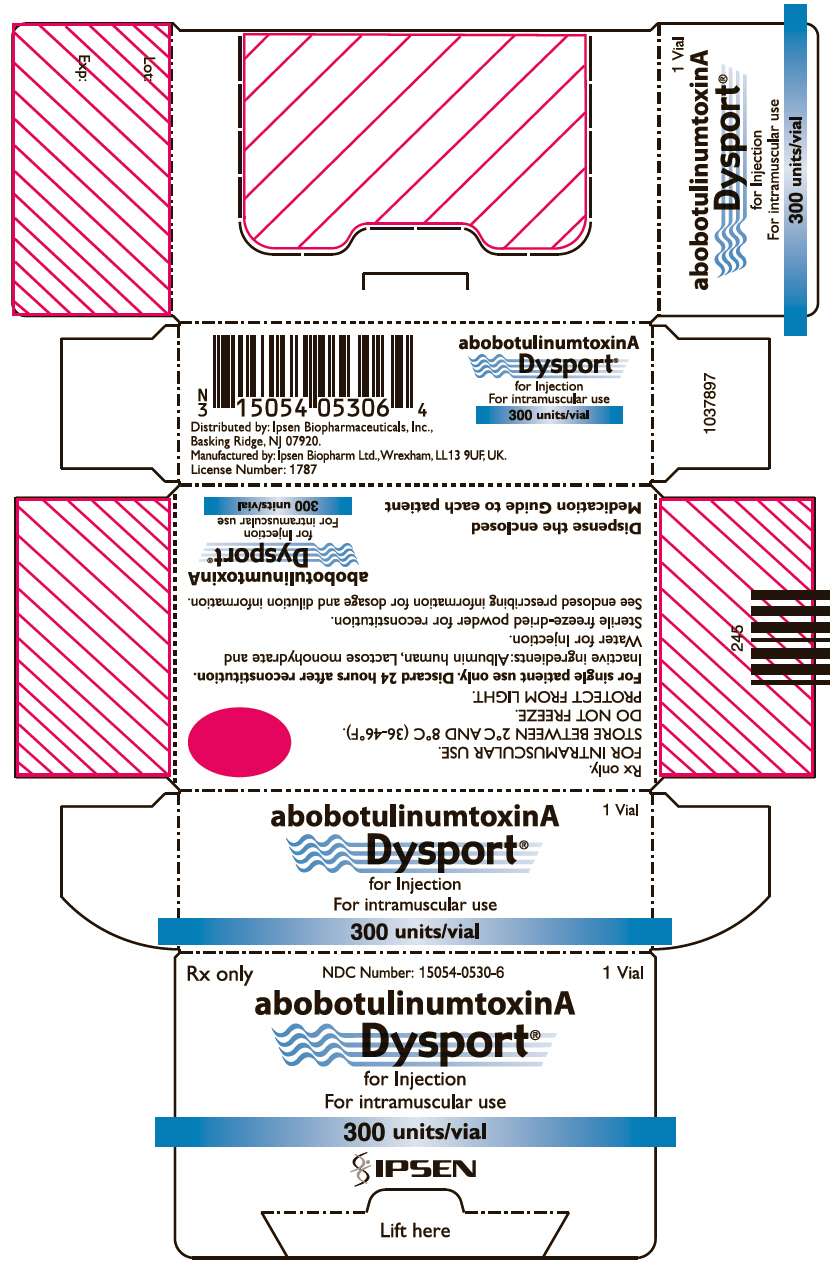
- PRINCIPAL DISPLAY PANEL - Vial Carton - 300 Units
FULL PRESCRIBING INFORMATION
Distant Spread of Toxin Effect
Postmarketing reports indicate that the effects of DYSPORT® and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses, including spasticity in children and adults, and in approved indications, cases of spread of effect have been reported at doses comparable to those used to treat cervical dystonia and at lower doses.
1 INDICATIONS AND USAGE
1.1 Cervical Dystonia
DYSPORT® (abobotulinumtoxinA) is an acetylcholine release inhibitor and a neuromuscular blocking agent indicated for the treatment of adults with cervical dystonia to reduce the severity of abnormal head position and neck pain in both toxin-naïve and previously treated patients.
1.2 Glabellar Lines
DYSPORT® (abobotulinumtoxinA) is an acetylcholine release inhibitor and a neuromuscular blocking agent indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with procerus and corrugator muscle activity in adult patients < 65 years of age.
2 DOSAGE AND ADMINISTRATION
The potency Units of DYSPORT ® are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of DYSPORT ® cannot be compared to or converted into units of any other botulinum toxin products assessed with any other specific assay method [see Description (11) ].
Reconstitution instructions are specific for each of the 300 Unit vial and the 500 Unit vial. These volumes yield concentrations specific for the use for each indication.
2.1 Cervical Dystonia
The recommended initial dose of DYSPORT® for the treatment of cervical dystonia is 500 Units given intramuscularly as a divided dose among affected muscles in patients with or without a history of prior treatment with botulinum toxin. (A description of the average DYSPORT® dose and percentage of total dose injected into specific muscles in the pivotal clinical trials can be found in Table 5 of Section 14.1, Clinical Studies – Cervical Dystonia.) Limiting the dose injected into the sternocleidomastoid muscle may reduce the occurrence of dysphagia. Clinical studies with DYSPORT® in cervical dystonia suggest that the peak effect occurs between two and four weeks after injection. Simultaneous EMG-guided application of DYSPORT® may be helpful in locating active muscles not identified by physical examination alone.
Dose Modification
Where dose modification is necessary for the treatment of cervical dystonia, uncontrolled open-label studies suggest that dose adjustment can be made in 250 Unit steps according to the individual patient's response, with re-treatment every 12 weeks or longer, as necessary, based on return of clinical symptoms. Uncontrolled open-label studies also suggest that the total dose administered in a single treatment should be between 250 Units and 1000 Units. Re-treatment, if needed, should not occur in intervals of less than 12 weeks. Doses above 1000 Units have not been systematically evaluated.
2.1.1 Special Populations
Adults and elderly
The starting dose of 500 Units recommended for cervical dystonia is applicable to adults of all ages [see Use in Specific Populations (8.5) ].
Children
The safety and effectiveness of DYSPORT® in the treatment of cervical dystonia in pediatric patients less than 18 years of age has not been assessed [see Warnings and Precautions (5.2) ].
2.1.2 Instructions for Preparation and Administration
DYSPORT® is supplied as a single-use vial. Each 500 Unit vial of DYSPORT® is to be reconstituted with 1 mL of 0.9% Sodium Chloride Injection USP (without preservative) to yield a solution of 500 Units per mL. Each 300 Unit vial of DYSPORT ® is to be reconstituted with 0.6 mL of 0.9% Sodium Chloride Injection USP (without preservative) to yield a solution equivalent to 250 Units per 0.5 mL.
Using an appropriately sized sterile syringe, needle and aseptic technique, draw up 1.0 mL or 0.6 mL of sterile, 0.9% Sodium Chloride Injection USP (without preservative) for 500 and 300 Unit vials, respectively. Insert the needle into the DYSPORT® vial. The partial vacuum will begin to pull the saline into the vial. Any remaining required saline should be expressed into the vial manually. Do not use the vial if no vacuum is observed. Swirl gently to dissolve. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Reconstituted DYSPORT® should be a clear, colorless solution, free of particulate matter, otherwise it should not be injected.
Expel any air bubbles in the syringe barrel. Remove the needle used to reconstitute the product and attach an appropriately sized new sterile needle.
Once reconstituted, DYSPORT® should be stored in a refrigerator at 2–8°C (36–46°F) protected from light and used within 24 hours. Do not freeze reconstituted DYSPORT®. Discard the vial and needle in accordance with local regulations.
2.2 Glabellar Lines
The dose of DYSPORT® for the treatment of glabellar lines is a total of 50 Units given intramuscularly in five equal aliquots of 10 Units each to achieve clinical effect (see Figure 1 ).
2.2.1 Special Populations
Adults
A total dose of 50 Units of DYSPORT®, in five equal aliquots, should be administered to achieve clinical effect.
The clinical effect of DYSPORT® may last up to four months. Repeat dose clinical studies demonstrated continued efficacy with up to four repeated administrations. It should be administered no more frequently than every three months. When used for re-treatment, DYSPORT® should be reconstituted and injected using the same techniques as the initial treatment.
Children
DYSPORT® for glabellar lines is not recommended for use in pediatric patients less than 18 years of age [see Warnings and Precautions (5.2) ].
2.2.2 Instructions for Preparation and Administration
DYSPORT® is supplied as a single-use vial. Each 300 Unit vial of DYSPORT® is to be reconstituted with 2.5 mL of 0.9% Sodium Chloride Injection USP (without preservative) prior to injection. The concentration of the resulting solution will be 10 Units per 0.08 mL to be delivered in five equally divided aliquots of 0.08 mL each. DYSPORT® may also be reconstituted with 1.5 mL of 0.9% Sodium Chloride Injection USP (without preservative) for a solution of 10 Units per 0.05 mL to be delivered in five equally divided aliquots of 0.05 mL each.
Using an appropriately sized sterile syringe, needle and aseptic technique, draw up 2.5 mL or 1.5 mL of 0.9% Sodium Chloride Injection USP (without preservative). Insert the needle into the DYSPORT® vial. The partial vacuum will begin to pull the saline into the vial. Any remaining required saline should be expressed into the vial manually. Do not use the vial if no vacuum is observed. Swirl gently to dissolve. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Reconstituted DYSPORT® should be a clear, colorless solution, free of particulate matter otherwise it should not be injected.
Draw a single patient dose of DYSPORT® into a sterile syringe. Expel any air bubbles in the syringe barrel. Remove the needle used to reconstitute the product and attach a 30 gauge needle.
Once reconstituted, DYSPORT® should be stored in a refrigerator at 2–8°C (36–46°F) protected from light and used within 24 hours. Do not freeze reconstituted DYSPORT®. Discard the vial and needle in accordance with local regulations.
2.2.3 Injection Technique
Glabellar facial lines arise from the activity of the lateral corrugator and vertical procerus muscles. These can be readily identified by palpating the tensed muscle mass while having the patient frown. The corrugator depresses the skin creating a "furrowed" vertical line surrounded by tensed muscle (i.e., frown lines). The location, size, and use of the muscles vary markedly among individuals. Physicians administering DYSPORT ® must understand the relevant neuromuscular and/or orbital anatomy of the area involved and any alterations to the anatomy due to prior surgical procedures.
Risk of ptosis can be mitigated by careful examination of the upper lid for separation or weakness of the levator palpebrae muscle (true ptosis), identification of lash ptosis, and evaluation of the range of lid excursion while manually depressing the frontalis to assess compensation.
In order to reduce the complication of ptosis, the following steps should be taken:
- Avoid injection near the levator palpebrae superioris, particularly in patients with larger brow depressor complexes.
- Medial corrugator injections should be placed at least 1 centimeter above the bony supraorbital ridge.
- Ensure the injected volume/dose is accurate and where feasible kept to a minimum.
- Do not inject toxin closer than 1 centimeter above the central eyebrow.
To inject DYSPORT®, advance the needle through the skin into the underlying muscle while applying finger pressure on the superior medial orbital rim. Inject patients with a total of 50 Units in five equally divided aliquots. Using a 30 gauge needle, inject 10 Units of DYSPORT® into each of five sites, two in each corrugator muscle, and one in the procerus muscle (see Figure 1 ).
Figure 1
3 DOSAGE FORMS AND STRENGTHS
3.1 Cervical Dystonia
DYSPORT® is supplied as:
- a single-use, sterile 500 Unit vial for reconstitution with 1 mL of 0.9% Sodium Chloride Injection USP (without preservative) to yield a solution of 500 Units per mL.
- a single-use, sterile 300 Unit vial for reconstitution with 0.6 mL of 0.9% Sodium Chloride Injection USP (without preservative) to yield a solution equivalent to 250 Units per 0.5 mL.
3.2 Glabellar Lines
DYSPORT® is supplied as:
- a single-use, sterile 300 Unit vial for reconstitution with 0.9% Sodium Chloride Injection USP (without preservative). DYSPORT ® may be reconstituted with either 2.5 mL to yield a solution of 10 Units per 0.08 mL or with 1.5 mL to yield a solution of 10 Units per 0.05 mL.
4 CONTRAINDICATIONS
DYSPORT® is contraindicated in patients with known hypersensitivity to any botulinum toxin preparation or to any of the components in the formulation [see Adverse Reactions (6.1), Description (11) ].
This product may contain trace amounts of cow's milk protein. Patients known to be allergic to cow's milk protein should not be treated with DYSPORT®.
DYSPORT® is contraindicated for use in patients with infection at the proposed injection site(s).
5 WARNINGS AND PRECAUTIONS
5.1 Lack of Interchangeability between Botulinum Toxin Products
The potency Units of DYSPORT® are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of DYSPORT® cannot be compared to or converted into units of any other botulinum toxin products assessed with any other specific assay method [see Description (11) ].
5.2 Spread of Toxin Effect
Post-marketing safety data from DYSPORT ® and other approved botulinum toxins suggest that botulinum toxin effects may, in some cases, be observed beyond the site of local injection. The symptoms are consistent with the mechanism of action of botulinum toxin and may include asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death related to spread of toxin effects. The risk of the symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, and particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses, including spasticity in children and adults, and in approved indications, symptoms consistent with spread of toxin effect have been reported at doses comparable to or lower than doses used to treat cervical dystonia.
5.3 Dysphagia and Breathing Difficulties in Treatment of Cervical Dystonia
Treatment with DYSPORT ® and other botulinum toxin products can result in swallowing or breathing difficulties. Patients with pre-existing swallowing or breathing difficulties may be more susceptible to these complications. In most cases, this is a consequence of weakening of muscles in the area of injection that are involved in breathing or swallowing. When distant effects occur, additional respiratory muscles may be involved [see Warnings and Precautions (5.2) ].
Deaths as a complication of severe dysphagia have been reported after treatment with botulinum toxin. Dysphagia may persist for several weeks, and require use of a feeding tube to maintain adequate nutrition and hydration. Aspiration may result from severe dysphagia and is a particular risk when treating patients in whom swallowing or respiratory function is already compromised.
Treatment of cervical dystonia with botulinum toxins may weaken neck muscles that serve as accessory muscles of ventilation. This may result in a critical loss of breathing capacity in patients with respiratory disorders who may have become dependent upon these accessory muscles. There have been post-marketing reports of serious breathing difficulties, including respiratory failure, in cervical dystonia patients.
Patients treated with botulinum toxin may require immediate medical attention should they develop problems with swallowing, speech or respiratory disorders. These reactions can occur within hours to weeks after injection with botulinum toxin [see Warnings and Precautions (5.2), Adverse Reactions (6.1), Clinical Pharmacology (12.2) ].
5.4 Facial Anatomy in the Treatment of Glabellar Lines
Caution should be exercised when administering DYSPORT ® to patients with surgical alterations to the facial anatomy, excessive weakness or atrophy in the target muscle(s), marked facial asymmetry, inflammation at the injection site(s), ptosis, excessive dermatochalasis, deep dermal scarring, thick sebaceous skin [see Dosage and Administration (2.2.3) ] or the inability to substantially lessen glabellar lines by physically spreading them apart [see Clinical Studies (14.2) ].
Do not exceed the recommended dosage and frequency of administration of DYSPORT ® . In clinical trials, subjects who received a higher dose of DYSPORT ® had an increased incidence of eyelid ptosis.
5.5 Pre-existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis or neuromuscular junction disorders (e.g., myasthenia gravis or Lambert-Eaton syndrome) should be monitored particularly closely when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including severe dysphagia and respiratory compromise from typical doses of DYSPORT ® [see Adverse Reactions (6.1) ].
5.6 Human Albumin
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) is also considered extremely remote. No cases of transmission of viral diseases or CJD have ever been reported for albumin.
5.7 Intradermal Immune Reaction
The possibility of an immune reaction when injected intradermally is unknown. The safety of DYSPORT ® for the treatment of hyperhidrosis has not been established.
6 ADVERSE REACTIONS
The following adverse reactions to DYSPORT ® are discussed in greater detail in other sections of the labeling.
- Hypersensitivity [see Contraindications (4) ]
- Dysphagia and Breathing Difficulties in Treatment of Cervical Dystonia [see Warnings and Precautions (5.3) ]
- Spread of Effects from Toxin [see Warnings and Precautions (5.2) ]
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates in other trials and may not reflect the rates observed in clinical practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating incidence rates.
Cervical Dystonia
The data described below reflect exposure to DYSPORT ® in 357 cervical dystonia patients in 6 studies. Of these, two studies were randomized, double-blind, single treatment, placebo controlled studies with subsequent optional open label treatment in which dose optimization (250 to 1000 Units per treatment) over the course of 5 treatment cycles was allowed.
The population was almost entirely Caucasian (99.2%) with a median age of 51 years (range 18–82 years). Most patients (86.6%) were less than 65 years of age; 58.4% were women.
Common Adverse Events
The most commonly reported adverse events (occurring in more than 5% of patients who received 500 Units of DYSPORT ® in the placebo controlled clinical trials) in cervical dystonia patients were muscular weakness, dysphagia, dry mouth, injection site discomfort, fatigue, headache, neck pain, musculoskeletal pain, dysphonia, injection site pain, and eye disorders (consisting of blurred vision, diplopia, and reduced visual acuity and accommodation). Most adverse events were reported as mild or moderate in severity. Other than injection site reactions, most adverse events became noticeable about one week after treatment and lasted several weeks.
The rates of adverse events were higher in the combined controlled and open-label experience than in the placebo-controlled trials.
During the clinical studies, two patients (<1%) experienced adverse events leading to withdrawal. One patient experienced disturbance in attention, eyelid disorder, feeling abnormal and headache, and one patient experienced dysphagia.
Table 1 compares the incidence of the most frequent treatment-emergent adverse events (TEAEs) from a single treatment cycle of 500 Units of DYSPORT ® compared to placebo [see Clinical Studies (14.1) ].
| Double-blind Phase | ||
|---|---|---|
| System Organ Class Preferred Term |
DYSPORT® 500 Units (N=173) |
Placebo (N=182) |
| % | % | |
| Any TEAE | 61 | 51 |
| General disorders and administration site conditions | 30 | 23 |
| Injection site discomfort | 13 | 8 |
| Fatigue | 12 | 10 |
| Injection site pain | 5 | 4 |
| Musculoskeletal and connective tissue disorders | 30 | 18 |
| Muscular weakness | 16 | 4 |
| Musculoskeletal pain | 7 | 3 |
| Gastrointestinal disorders | 28 | 15 |
| Dysphagia | 15 | 4 |
| Dry mouth | 13 | 7 |
| Nervous system disorders | 16 | 13 |
| Headache | 11 | 9 |
| Infections and infestations | 13 | 9 |
| Respiratory, thoracic and mediastinal disorders | 12 | 8 |
| Dysphonia | 6 | 2 |
|
Eye Disorders |
7 | 2 |
Dose-response relationships for common adverse events in a randomized multiple fixed-dose study in which the total dose was divided between two muscles (the sternocleidomastoid and splenius capitis) are shown in Table 2.
| System Organ Class Preferred Term |
DYSPORT® Dose | |||
|---|---|---|---|---|
| Placebo | 250 Units | 500 Units | 1000 Units | |
| Any Adverse Event | 30% | 37% | 65% | 83% |
| Dysphagia | 5% | 21% | 29% | 39% |
| Dry Mouth | 10% | 21% | 18% | 39% |
| Muscular Weakness | 0% | 11% | 12% | 56% |
| Injection Site Discomfort | 10% | 5% | 18% | 22% |
| Dysphonia | 0% | 0% | 18% | 28% |
| Facial Paresis | 0% | 5% | 0% | 11% |
| Eye Disorders | 0% | 0% | 6% | 17% |
Injection Site Reactions
Injection site discomfort and injection site pain were common adverse events following DYSPORT ® administration. These events were mainly of mild or moderate intensity.
Less Common Adverse Events
The following selected adverse events were reported less frequently (<5%).
Breathing Difficulties
Breathing difficulties were reported by approximately 3% of patients following DYSPORT ® administration and in 1% of placebo patients in clinical trials during the double-blind phase. These consisted mainly of dyspnea and were generally mild in intensity. The median time to onset from last dose of DYSPORT ® was approximately one week, and the median duration was approximately three weeks.
Other selected adverse events with incidences of less than 5% in the DYSPORT ® 500 Units group in the double-blind phase of clinical trials included dizziness in 3.5% of DYSPORT ® -treated subjects and 1% of placebo-treated subjects, and muscle atrophy in 1% of DYSPORT ® -treated subjects and in none of the placebo-treated subjects.
Laboratory Findings
Subjects treated with DYSPORT ® exhibited a small increase from baseline (0.23 mol/L) in mean blood glucose relative to placebo-treated subjects. This was not clinically significant among subjects in the development program but could be a factor in patients whose diabetes is difficult to control.
Electrocardiographic Findings
ECG measurements were only recorded in a limited number of subjects in an open-label study without a placebo or active control. This study showed a statistically significant reduction in heart rate compared to baseline, averaging about three beats per minute, observed thirty minutes after injection.
Glabellar Lines
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not be predictive of rates observed in practice.
In placebo-controlled clinical trials of DYSPORT ® , the most frequently reported adverse events (≥2%) following injection of DYSPORT ® were nasopharyngitis, headache, injection site pain, injection site reaction, upper respiratory tract infection, eyelid edema, eyelid ptosis, sinusitis and nausea.
Table 3 reflects exposure to DYSPORT ® in 398 subjects aged 19 to 75 who were evaluated in the randomized, placebo-controlled clinical studies that assessed the use of DYSPORT ® for the temporary improvement in the appearance of glabellar lines [see Clinical Studies (14) ]. Adverse events of any cause were reported for 48% of the DYSPORT ® -treated subjects and 33% of the placebo-treated subjects. Treatment-emergent adverse events were generally mild to moderate in severity.
| Adverse Events by Body System | DYSPORT®
n=398 (%) |
Placebo n=496 (%)  |
|---|---|---|
| Any Treatment-emergent Adverse Event | 191 (48) | 163 (33) |
| Eye Disorders | ||
| Eyelid Edema | 8 (2) | 0 |
| Eyelid Ptosis | 6 (2) | 1 (<1) |
| Gastrointestinal Disorders | ||
| Nausea | 6 (2) | 5 (1) |
| General Disorders and Administration Site Conditions | ||
| Injection Site Pain | 11 (3) | 8 (2) |
| Injection Site Reaction | 12 (3) | 2 (<1) |
| Infections and Infestations | ||
| Nasopharyngitis | 38 (10) | 21 (4) |
| Upper Respiratory Tract Infection | 12 (3) | 9 (2) |
| Sinusitis | 8 (2) | 6 (1) |
| Investigations | ||
| Blood Urine Present | 6 (2) | 1 (<1) |
| Nervous System Disorders | ||
| Headache | 37 (9) | 23 (5) |
In the overall safety database, where some subjects received up to twelve treatments with DYSPORT ® , adverse events were reported for 57% (1425/2491) of subjects. The most frequently reported of these adverse events were headache, nasopharyngitis, injection site pain, sinusitis, URI, injection site bruising, and injection site reaction (numbness, discomfort, erythema, tenderness, tingling, itching, stinging, warmth, irritation, tightness, swelling).
Adverse events that emerged after repeated injections in 2–3% of the population included bronchitis, influenza, pharyngolaryngeal pain, cough, contact dermatitis, injection site swelling, and injection site discomfort.
The incidence of eyelid ptosis did not increase in the long-term safety studies with multiple re-treatments at intervals ≥ three months. The majority of eyelid ptosis events were mild to moderate in severity and resolved over several weeks. [see Injection Technique (2.2.3) ].
6.2 Post-marketing Spontaneous Reports
There is extensive post-marketing experience outside the U.S. for the treatment of glabellar lines. Adverse reactions are reported voluntarily from a population of uncertain size; thus, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure. The following adverse reactions have been identified during post-marketing use: vertigo, eyelid ptosis, diplopia, vision blurred, photophobia, dysphagia, nausea, injection site reaction, malaise, influenza-like illness, hypersensitivity, sinusitis, amyotrophy, burning sensation, facial paresis, dizziness, headache, hypoesthesia, erythema, and excessive granulation tissue.
6.3 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity.
The incidence of antibody formation is highly dependent on the sensitivity and specificity of the assay. In addition, the observed incidence of antibody positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies across products in this class may be misleading.
Cervical Dystonia
About 3% of subjects developed antibodies (binding or neutralizing) over time with DYSPORT ® treatment. The significance of these antibodies is unknown since in the presence of binding and neutralizing antibodies some patients may continue to experience clinical benefit.
Glabellar Lines
Testing for antibodies to DYSPORT ® was performed for 1554 subjects who had up to nine cycles of treatment. Two subjects (0.13%) tested positive for binding antibodies at baseline. Three additional subjects tested positive for binding antibodies after receiving DYSPORT ® treatment. None of the subjects tested positive for neutralizing antibodies.
7 DRUG INTERACTIONS
No formal drug interaction studies have been conducted with DYSPORT ® .
Patients treated concomitantly with botulinum toxins and aminoglycosides or other agents interfering with neuromuscular transmission (e.g., curare-like agents) should be observed closely because the effect of the botulinum toxin may be potentiated. Use of anticholinergic drugs after administration of DYSPORT ® may potentiate systemic anticholinergic effects such as blurred vision.
The effect of administering different botulinum neurotoxin products at the same time or within several months of each other is unknown. Excessive weakness may be exacerbated by another administration of botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin.
Excessive weakness may also be exaggerated by administration of a muscle relaxant before or after administration of DYSPORT ® .
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
DYSPORT ® produced embryo-fetal toxicity when given to pregnant rats at doses similar to or greater than the maximum recommended human dose (MRHD) of 1000 Units on a body weight (Units/kg) basis.
In an embryo-fetal development study in which pregnant rats received intramuscular injections daily (2.2, 6.6, or 22 Units/kg on gestation days 6 through 17) or intermittently (44 Units/kg on gestation days 6 and 12 only) during organogenesis, increased early embryonic death was observed with both dosing schedules. The no-effect dose for embryo-fetal developmental toxicity was 2.2 Units/kg (one-tenth the MRHD on a body weight basis). Maternal toxicity was seen at 22 and 44 Units/kg. In a pre-and post-natal development study in which female rats received 6 weekly intramuscular injections (4.4, 11.1, 22.2, or 44 Units/kg) beginning on day 6 of gestation and continuing through parturition to weaning, an increase in stillbirths was observed at the highest dose, which was maternally toxic. The no-effect dose for pre- and post-natal developmental toxicity was 22.2 Units/kg (approximately equal to the MRHD on a body weight basis).
There are no adequate and well-controlled studies in pregnant women. DYSPORT ® should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
It is not known whether DYSPORT ® is excreted in human milk.
8.4 Pediatric Use
Cervical Dystonia
Safety and effectiveness in pediatric patients have not been established [see Warnings and Precautions (5.2) ].
Glabellar Lines
DYSPORT ® is not recommended for use in pediatric patients less than 18 years of age.
8.5 Geriatric Use
Cervical Dystonia
There were insufficient numbers of patients aged 65 and over in the clinical studies to determine whether they respond differently than younger patients. In general, elderly patients should be observed to evaluate their tolerability of DYSPORT ® , due to the greater frequency of concomitant disease and other drug therapy [see Dosage and Administration (2.1.1) ].
Glabellar Lines
Of the total number of subjects in the placebo-controlled clinical studies of DYSPORT ® , 8 (1%) were 65 and over. Efficacy was not observed in subjects 65 years and over [see Clinical Studies (14.2) ]. For the entire safety database of geriatric subjects, although there was no increase in the incidence of eyelid ptosis, geriatric subjects did have an increase in the number of ocular adverse events compared to younger subjects (11% vs. 5%) [see Dosage and Administration (2.2) ].
8.6 Ethnic Groups
Exploratory analyses in trials for glabellar lines in African-American subjects with Fitzpatrick skin types IV, V, or VI and in Hispanic subjects suggested that response rates at Day 30 were comparable to and no worse than the overall population.
10 OVERDOSAGE
Excessive doses of DYSPORT ® may be expected to produce neuromuscular weakness with a variety of symptoms. Respiratory support may be required where excessive doses cause paralysis of respiratory muscles. In the event of overdose, the patient should be medically monitored for symptoms of excessive muscle weakness or muscle paralysis [see Warnings and Precautions (5.2) ]. Symptomatic treatment may be necessary.
Symptoms of overdose are likely not to be present immediately following injection. Should accidental injection or oral ingestion occur, the person should be medically supervised for several weeks for signs and symptoms of excessive muscle weakness or paralysis.
There is no significant information regarding overdose from clinical studies in cervical dystonia. Doses exceeding 1000 Units of DYSPORT ® were rarely studied in clinical settings for any indication.
In the event of overdose, antitoxin raised against botulinum toxin is available from the Centers for Disease Control and Prevention (CDC) in Atlanta, GA. However, the antitoxin will not reverse any botulinum toxin-induced effects already apparent by the time of antitoxin administration. In the event of suspected or actual cases of botulinum toxin poisoning, please contact your local or state Health Department to process a request for antitoxin through the CDC. If you do not receive a response within 30 minutes, please contact the CDC directly at 770-488-7100. More information can be obtained at http://www.cdc.gov/ncidod/srp/drugs/drug-service.html.
11 DESCRIPTION
Botulinum toxin type A, the active ingredient in DYSPORT ® (abobotulinumtoxinA), is a purified neurotoxin type A complex produced by fermentation of the bacterium Clostridium botulinum type A, Hall Strain. It is purified from the culture supernatant by a series of precipitation, dialysis, and chromatography steps. The neurotoxin complex is composed of the neurotoxin, hemagglutinin proteins and non-toxin non-hemagglutinin protein.
DYSPORT ® is supplied in a single-use, sterile vial for reconstitution intended for intramuscular injection. Each vial contains 500 or 300 Units of lyophilized abobotulinumtoxinA, 125 micrograms human serum albumin and 2.5 mg lactose. DYSPORT ® may contain trace amounts of cow's milk proteins [see Contraindications (4) ].
One unit of DYSPORT ® corresponds to the calculated median lethal intraperitoneal dose (LD50) in mice. The method for performing the assay is specific to Ipsen's product DYSPORT ® . Due to differences in specific details such as vehicle, dilution scheme and laboratory protocols for various mouse LD50 assays, Units of biological activity of DYSPORT ® are not interchangeable with Units of any other botulinum toxin or any toxin assessed with any other specific assay method [see Dosage Forms and Strengths (3) ].
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
DYSPORT ® inhibits release of the neurotransmitter, acetylcholine, from peripheral cholinergic nerve endings. Toxin activity occurs in the following sequence: Toxin heavy chain mediated binding to specific surface receptors on nerve endings, internalization of the toxin by receptor mediated endocytosis, pH-induced translocation of the toxin light chain to the cell cytosol and cleavage of SNAP25 leading to intracellular blockage of neurotransmitter exocytosis into the neuromuscular junction. This accounts for the therapeutic utility of the toxin in diseases characterized by excessive efferent activity in motor nerves.
Recovery of transmission occurs gradually as the neuromuscular junction recovers from SNAP25 cleavage and as new nerve endings are formed.
12.2 Pharmacodynamics
The primary pharmacodynamic effect of DYSPORT ® is due to chemical denervation of the treated muscle resulting in a measurable decrease of the compound muscle action potential, causing a localized reduction of muscle activity.
12.3 Pharmacokinetics
Using currently available analytical technology, it is not possible to detect DYSPORT ® in the peripheral blood following intramuscular injection at the recommended doses.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenicity, Mutagenicity, Impairment of Fertility
Carcinogenicity
Studies to evaluate the carcinogenic potential of DYSPORT ® have not been conducted.
Mutagenicity
Genotoxicity studies have not been conducted for DYSPORT ® .
Impairment of Fertility
In a fertility and early embryonic development study in rats in which either males (2.9, 7.2, 14.5 or 29 Units/kg) or females (7.4, 19.7, 39.4 or 78.8 Units/kg) received weekly intramuscular injections prior to and after mating, dose-related increases in pre-implantation loss and reduced numbers of corpora lutea were noted in treated females. Failure to mate was observed in males that received the high dose. The no-effect dose for effects on fertility was 7.4 Units/kg in females and 14.5 Units/kg in males (approximately one-half and equal to, respectively, the maximum recommended human dose of 1000 Units on a body weight basis).
14 CLINICAL STUDIES
14.1 Cervical Dystonia
The efficacy of DYSPORT ® was evaluated in two well-controlled, randomized, double-blind, placebo controlled, single dose, parallel group studies in treatment-naïve cervical dystonia patients. The principal analyses from these trials provide the primary demonstration of efficacy involving 252 patients (121 on DYSPORT ® , 131 on placebo) with 36% male and 64% female. Ninety-nine percent of the patients were Caucasian.
In both placebo controlled studies (Study 1 and Study 2), a dose of 500 Units DYSPORT ® was given by intramuscular injection divided among two to four affected muscles. These studies were followed by long-term open label extensions that allowed titration in 250 Unit steps to doses in a range of 250 to 1000 Units, after the initial dose of 500 Units. In the extension studies, re-treatment was determined by clinical need after a minimum of 12 weeks. The median time to re-treatment was 14 weeks and 18 weeks for the 75th percentile.
The primary assessment of efficacy was based on the total Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) change from baseline at Week 4 for both studies. The scale evaluates the severity of dystonia, patient perceived disability from dystonia, and pain. The adjusted mean change from baseline in the TWSTRS total score was statistically significantly greater for the DYSPORT ® group than the placebo group at Weeks 4 in both studies (see Table 4 ).
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| DYSPORT®
500 Units N=55 |
Placebo N=61 |
DYSPORT®
500 Units N=37 |
Placebo N=43 |
|
| Baseline (week 0) | ||||
| Mean (SD) | 43.8 (8.0) | 45.8 (8.9) | 45.1 (8.7) | 46.2 (9.4) |
| Week 4 | ||||
| Mean (SD) | 30.0 (12.7) | 40.2 (11.8) | 35.2 (13.8) | 42.4 (12.2) |
Change from Baseline |
-15.6 (2.0) | -6.7 (2.0) | -9.6 (2.0) | -3.7 (1.8) |
| Treatment difference | -8.9 |
-5.9 |
||
| 95% confidence interval | [-12.9 to -4.7] | [-10.6 to -1.3] | ||
| Week 8 | ||||
| Mean (SD) | 29.3 (11.0) | 39.6 (13.5) | ||
Change from Baseline |
-14.7 (2.0) | -5.9 (2.0) | ||
| Treatment difference | -8.8 |
|||
| 95% confidence interval | [-12.9 to -4.7] | |||
Analyses by gender, weight, geographic region, underlying pain, cervical dystonia severity at baseline and history of treatment with botulinum toxin did not show any meaningful differences between groups.
Table 5 indicates the average DYSPORT ® dose, and percentage of total dose, injected into specific muscles in the pivotal clinical trials.
| Number of patients injected per muscle |
DYSPORT® Dose Injected | Percentage of the total DYSPORT® Dose Injected | |||
|---|---|---|---|---|---|
| Median [DYSPORT® Units] (min, max) |
75th percentile [DYSPORT® Units] |
Median [%] (min, max) |
75th percentile [%] |
||
| Sternocleidomastoid | 90 | 125 Units (50, 350) |
150 Units | 26.5 % (10, 70) |
30.0 % |
| Splenius capitis | 85 | 200 Units (75, 450) |
250 Units | 40.0 % (15, 90) |
50.0 % |
| Trapezius | 50 | 102.6 Units (50, 300) |
150 Units | 20.6 % (10, 60) |
30.0 % |
| Levator scapulae | 35 | 105.3 Units (50, 200) |
125 Units | 21.1 % (10, 40) |
25.0 % |
| Scalenus (medius and anterior) | 26 | 115.5 Units (50, 300) |
150 Units | 23.1 % (10, 60) |
30.0 % |
| Semispinalis capitis | 21 | 131.6 Units (50, 250) |
175 Units | 29.4 % (10, 50) |
35.0 % |
| Longissimus | 3 | 150 Units (100, 200) |
200 Units | 30.0 % (20, 40) |
40.0 % |
14.2 Glabellar Lines
Three double-blind, randomized, placebo-controlled, clinical studies evaluated the efficacy of DYSPORT® for use in the temporary improvement of the appearance of moderate to severe glabellar lines. These three studies enrolled healthy adults (ages 19-75) with glabellar lines of at least moderate severity at maximum frown. Subjects were excluded if they had marked ptosis, deep dermal scarring, or a substantial inability to lessen glabellar lines, even by physically spreading them apart. The subjects in these studies received either DYSPORT® or placebo. The total dose was delivered in equally divided aliquots to specified injection sites (see Figure 1 ).
Investigators and subjects assessed efficacy at maximum frown by using a 4-point scale (none, mild, moderate, severe).
Overall treatment success was defined as post-treatment glabellar line severity of none or mild with at least 2 grade improvement from Baseline for the combined investigator and subject assessments (composite assessment) on Day 30 (see Table 6 ). Additional endpoints for each of the studies were post-treatment glabellar line severity of none or mild with at least a 1 grade improvement from Baseline for the separate investigator and subject assessments on Day 30.
After completion of the randomized studies, subjects were offered participation in a two-year, open-label re-treatment study to assess the safety of multiple treatments.
| 2 Grade Improvement | ||
|---|---|---|
| Study | DYSPORT®
n/N (%) |
Placebo n/N (%) |
| GL-1 | 58/105 (55%) | 0/53 (0%) |
| GL-2 | 37/71 (52%) | 0/71 (0%) |
| GL-3 | 120/200 (60%) | 0/100 (0%) |
Treatment with DYSPORT ® reduced the severity of glabellar lines for up to four months.
Study GL-1
Study GL-1 was a single dose, double-blind, multi-center, randomized, placebo-controlled study in which 158 previously untreated subjects received either placebo or 50 Units of DYSPORT®, administered in five aliquots of 10 Units (see Figure 1 ). Subjects were followed for 180 days. The mean age was 43 years; most of the subjects were women (85%), and predominantly Caucasian (49%) or Hispanic (47%). At Day 30, 55% of DYSPORT®-treated subjects achieved treatment success: a composite 2 grade improvement of glabellar line severity at maximum frown (see Table 6 ).
In study GL-1, the reduction of glabellar line severity at maximum frown was greater at Day 30 in the DYSPORT® group compared to the placebo group as assessed by both Investigators and subjects (see Table 7 ).
| Investigator's Assessment | Subject's Assessment | |||
|---|---|---|---|---|
| Day | DYSPORT®
N=105 |
Placebo N=53 |
DYSPORT®
N=105 |
Placebo N=53 |
| 14 | 90% 95 |
17% 9 |
77% 81 |
9% 5 |
| 30 | 88% 92 |
4% 2 |
74% 78 |
9% 5 |
| 60 | 64% 67 |
2% 1 |
60% 63 |
6% 3 |
| 90 | 43% 45 |
6% 3 |
36% 38 |
6% 3 |
| 120 | 23% 24 |
4% 2 |
19% 20 |
6% 3 |
| 150 | 9% 9 |
2% 1 |
8% 8 |
4% 2 |
| 180 | 6% 6 |
0% 0 |
7% 7 |
8% 4 |
Study GL-2
Study GL-2 was a repeat dose, double-blind, multi-center, placebo-controlled, randomized study. The study was initiated with two or three open-label treatment cycles of 50 Units of DYSPORT ® administered in five aliquots of 10 Units DYSPORT ® (see Figure 1 ). After the open-label treatments, subjects were randomized to receive either placebo or 50 Units of DYSPORT ® . Subjects could have received up to four treatments through the course of the study. Efficacy was assessed in the final randomized treatment cycle. The study enrolled 311 subjects into the first treatment cycle and 142 subjects were randomized into the final treatment cycle. Overall, the mean age was 47 years; most of the subjects were women (86%) and predominantly Caucasian (80%).
At Day 30, 52% of DYSPORT ®-treated subjects achieved treatment success: a composite 2 grade improvement of glabellar line severity at maximum frown (see Table 6 ).
The proportion of responders in the final treatment cycle was comparable to the proportion of responders in all prior treatment cycles.
After the final repeat treatment with DYSPORT ® , the reduction of glabellar line severity at maximum frown was greater at Day 30 in the DYSPORT ® group compared to the placebo group as assessed by both Investigators and subjects (see Table 8 ).
| Investigator's Assessment | Subject's Assessment | |||
|---|---|---|---|---|
| Day | DYSPORT®
N=71 |
Placebo N=71 |
DYSPORT®
N=71 |
Placebo N=71 |
| 30 | 85% 60 |
4% 3 |
79% 56 |
1% 1 |
Study GL-3
Study GL-3 was a single dose, double-blind, multi-center, randomized, placebo-controlled study in which 300 previously untreated subjects received either placebo or 50 Units of DYSPORT®, administered in five aliquots of 10 Units (see Figure 1 ). Subjects were followed for 150 days. The mean age was 44 years; most of the subjects were women (87%), and predominantly Caucasian (75%) or Hispanic (18%).
At Day 30, 60% of DYSPORT®-treated subjects achieved treatment success: a composite 2 grade improvement of glabellar line severity at maximum frown (see Table 6 ).
In study GL-3, the reduction of glabellar line severity at maximum frown was greater at Day 30 in the DYSPORT® group compared to the placebo group as assessed by both Investigators and subjects (see Table 9 ).
| Investigator's Assessment | Subject's Assessment | |||
|---|---|---|---|---|
| Day | DYSPORT®
N=200 |
Placebo N=100 |
DYSPORT®
N=200 |
Placebo N=100 |
| 14 | 83% 166 |
5% 5 |
83% 165 |
2% 2 |
| 30 | 86% 171 |
0% 0 |
82% 163 |
2% 2 |
| 60 | 75% 150 |
1% 1 |
65% 130 |
4% 4 |
| 90 | 51% 102 |
1% 1 |
46% 91 |
2% 2 |
| 120 | 29% 58 |
1% 1 |
31% 61 |
3% 3 |
| 150 | 16% 32 |
1% 1 |
16% 31 |
3% 3 |
Geriatric Subjects
In GL1, GL2, and GL3, there were 8 subjects aged 65 and older who were randomized to DYSPORT ® 50 Units in 5 equal aliquots of 10 Units (4) or placebo (4). None of the geriatric DYSPORT ® subjects were a treatment success at maximum frown at Day 30.
16 HOW SUPPLIED/STORAGE AND HANDLING
DYSPORT ® for Injection is supplied in a sterile, single-use, 3 mL glass vial. DYSPORT ® must be stored under refrigeration at 2–8°C (36–46°F). Protect from light.
Administer DYSPORT ® within 24 hours of reconstitution; during this period reconstituted DYSPORT ® should be stored under refrigeration at 2–8°C (36–46°F). Do not freeze after reconstitution.
Do not use after the expiration date on the vial. All vials, including expired vials, or equipment used with DYSPORT ® should be disposed of carefully as is done with all medical waste.
DYSPORT ® contains a unique hologram on the carton. If you do not see the hologram, do not use the product. Instead contact 877-397-7671.
Cervical Dystonia
-
-
-
-
-
-
-
-
-
Glabellar Lines
-
-
17 PATIENT COUNSELING INFORMATION
The physician should provide a copy of the FDA-Approved Patient Medication Guide and review the contents with the patient. Patients should be advised to inform their doctor or pharmacist if they develop any unusual symptoms (including difficulty with swallowing, speaking or breathing), or if any known symptom persists or worsens.
Patients should be counseled that if loss of strength, muscle weakness, blurred vision or drooping eyelids occur, they should avoid driving a car or engaging in other potentially hazardous activities.
Manufactured by:
Ipsen Biopharm Ltd.
Wrexham, LL13 9UF, UK
Distributed by:
Ipsen Biopharmaceuticals, Inc.
Basking Ridge, NJ 07920
And
Medicis Aesthetics Inc.
a wholly owned subsidiary of Medicis Pharmaceutical Corporation
Scottsdale, AZ 85256
MEDICATION GUIDE
DYSPORT® (DIS-port)
(abobotulinumtoxinA)
Injection
Read the Medication Guide that comes with DYSPORT® before you start using it and each time DYSPORT® is given to you. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. You should share this information with your family members and caregivers.
What is the most important information I should know about DYSPORT®?
DYSPORT® may cause serious side effects that can be life threatening including:
- Problems breathing or swallowing
- Spread of toxin effects
These problems can happen within hours, or days to weeks after an injection of DYSPORT®. Call your doctor or get medical help right away if you have any of these problems after treatment with DYSPORT®:
-
1. Problems swallowing, speaking, or breathing. These problems can happen within hours, or days to weeks after an injection of DYSPORT® usually because the muscles that you use to breathe and swallow can become weak after the injection. Death can happen as a complication if you have severe problems with swallowing or breathing after treatment with DYSPORT®.- People with certain breathing problems may need to use muscles in their neck to help them breathe. These patients may be at greater risk for serious breathing problems with DYSPORT®.
- Swallowing problems may last for several weeks. People who can not swallow well may need a feeding tube to receive food and water. If swallowing problems are severe, food or liquids may go into your lungs. People who already have swallowing or breathing problems before receiving DYSPORT® have the highest risk of getting these problems.
-
2. Spread of toxin effects. In some cases, the effect of botulinum toxin may affect areas of the body away from the injection site and cause symptoms of a serious condition called botulism. The symptoms of botulism include:- loss of strength and muscle weakness all over the body
- double vision
- blurred vision and drooping eyelids
- hoarseness or change or loss of voice (dysphonia)
- trouble saying words clearly (dysarthria)
- loss of bladder control
- trouble breathing
- trouble swallowing
These symptoms can happen within hours, or days to weeks after you receive an injection of DYSPORT®. These problems could make it unsafe for you to drive a car or do other dangerous activities. See "What should I avoid while receiving DYSPORT®?".
What is DYSPORT®?
DYSPORT® is a prescription medicine that is injected into muscles and used:
- to treat the abnormal head position and neck pain that happens with cervical dystonia (CD) in adults
- to improve the look of moderate to severe frown lines between the eyebrows (glabellar lines) in adults younger than 65 years of age for a short period of time (temporary)
CD is caused by muscle spasms in the neck. These spasms cause abnormal position of the head and often neck pain. After DYSPORT® is injected into muscles, those muscles are weakened for up to 12 to 16 weeks or longer. This may help lessen your symptoms.
Frown lines (wrinkles) happen because the muscles that control facial expression are used often (muscle tightening over and over). After DYSPORT® is injected into the muscles that control facial expression, the medicine stops the tightening of these muscles for up to 4 months.
It is not known whether DYSPORT® is safe or effective in children under 18 years of age.
It is not known whether DYSPORT® is safe or effective for the treatment of other types of muscle spasms. It is not known whether DYSPORT® is safe or effective for the treatment of other wrinkles.
Who should not take DYSPORT®?
Do not take DYSPORT® if you:
- are allergic to DYSPORT® or any of the ingredients in DYSPORT®. See the end of this Medication Guide for a list of ingredients in DYSPORT®
- are allergic to cow's milk protein
- had an allergic reaction to any other botulinum toxin product such as Myobloc® (rimabotulinumtoxinB)

All trademarks are the property of their respective owners , Botox®(onabotulinumtoxinA)
All trademarks are the property of their respective owners , or Xeomin®(incobotulinumtoxinA)
All trademarks are the property of their respective owners . - have a skin infection at the planned injection site
What should I tell my doctor before taking DYSPORT®?
Tell your doctor about all your medical conditions, including if you:
- have a disease that affects your muscles and nerves (such as amyotrophic lateral sclerosis [ALS or Lou Gehrig's disease], myasthenia gravis or Lambert-Eaton syndrome). See "What is the most important information I should know about DYSPORT® ?"
- have allergies to any botulinum toxin product
- had any side effect from any botulinum toxin product in the past
- have or have had a breathing problem, such as asthma or emphysema
- have or have had swallowing problems
- have or have had bleeding problems
- have diabetes
- have or have had a slow heart beat or other problem with your heart rate or rhythm
- have plans to have surgery
- had surgery on your face
- have weakness of your forehead muscles (such as trouble raising your eyebrows)
- have drooping eyelids
- have any other change in the way your face normally looks
- are pregnant or plan to become pregnant. It is not known if DYSPORT® can harm your unborn baby
- are breast-feeding or planning to breast-feed. It is not known if DYSPORT® passes into breast milk
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins and herbal products. Using DYSPORT® with certain other medicines may cause serious side effects. Do not start any new medicines until you have told your doctor that you have received DYSPORT® in the past.
Especially tell your doctor if you:
- have received any other botulinum toxin product in the last four months
- have received injections of botulinum toxin, such as Myobloc® (rimabotulinumtoxinB)

All trademarks are the property of their respective owners , Botox® (onabotulinumtoxinA)
All trademarks are the property of their respective owners or Xeomin® (incobotulinumtoxinA)
All trademarks are the property of their respective owners in the past; be sure your doctor knows exactly which product you received - have recently received an antibiotic by injection
- take muscle relaxants
- take an allergy or cold medicine
- take a sleep medicine
Ask your doctor if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist each time you get a new medicine.
How should I take DYSPORT®?
- DYSPORT® is an injection that your doctor will give you
- DYSPORT® is injected into the affected muscles
- Your doctor may give you another dose of DYSPORT® after 12 weeks or longer, if it is needed
- If you are being treated for CD, your doctor may change your dose of DYSPORT®, until you and your doctor find the best dose for you
- The dose of DYSPORT® is not the same as the dose of any other botulinum toxin product
What should I avoid while taking DYSPORT®?
DYSPORT® may cause loss of strength or general muscle weakness, blurred vision, or drooping eyelids within hours to weeks of taking DYSPORT®. If this happens, do not drive a car, operate machinery, or do other dangerous activities. See "What is the most important information I should know about DYSPORT®?"
What are the possible side effects of DYSPORT®?
DYSPORT® can cause serious side effects. See "What is the most important information I should know about DYSPORT®?"
Other side effects of DYSPORT® include:
- dry mouth
- injection site discomfort or pain
- tiredness
- headache
- neck pain
- muscle pain
- eye problems: double vision, blurred vision, decreased eyesight, problems with focusing the eyes (accommodation), drooping eyelids, swelling of the eyelids
- allergic reactions. Symptoms of an allergic reaction to DYSPORT® may include: itching, rash, red itchy welts, wheezing, asthma symptoms, or dizziness or feeling faint. Tell your doctor or get medical help right away if you get wheezing or asthma symptoms, or if you get dizzy or faint
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of DYSPORT®. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about DYSPORT®:
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
This Medication Guide summarizes the most important information about DYSPORT®. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about DYSPORT® that is written for healthcare professionals. For more information about DYSPORT® call 877-397-7671 or go to www.dysport.com or www.DysportUSA.com.
What are the ingredients in DYSPORT®?
Active ingredient: (botulinum toxin Type A)
Inactive ingredients: human albumin, and lactose. DYSPORT® may contain cow's milk protein.
Revised February 2012
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Distributed by:
Ipsen Biopharmaceuticals, Inc.
Basking Ridge, NJ 07920
and
Medicis Aesthetics Inc.,
a wholly owned subsidiary of Medicis Pharmaceutical Corporation
Scottsdale, AZ 85256
PRINCIPAL DISPLAY PANEL - Vial Carton - 500 Units
Rx only
NDC Number: 15054-0500-1
1 Vial
abobotulinumtoxinA
Dysport ®
for Injection
For intramuscular use
500 units/vial
IPSEN

PRINCIPAL DISPLAY PANEL - Vial Carton - 300 Units
Rx only
NDC Number: 15054-0530-6
1 Vial
abobotulinumtoxinA
Dysport ®
for Injection
For intramuscular use
300 units/vial
IPSEN
DysportBotulinum Toxin Type A INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DysportBotulinum Toxin Type A INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||