EFFET PARFAIT
EFFET PARFAIT natural finish moisturizing foundation SPF 25
FULL PRESCRIBING INFORMATION: CONTENTS*
- EFFET PARFAIT Uses
- Warnings
- Directions
- EFFET PARFAIT Other information
- Inactive ingredients
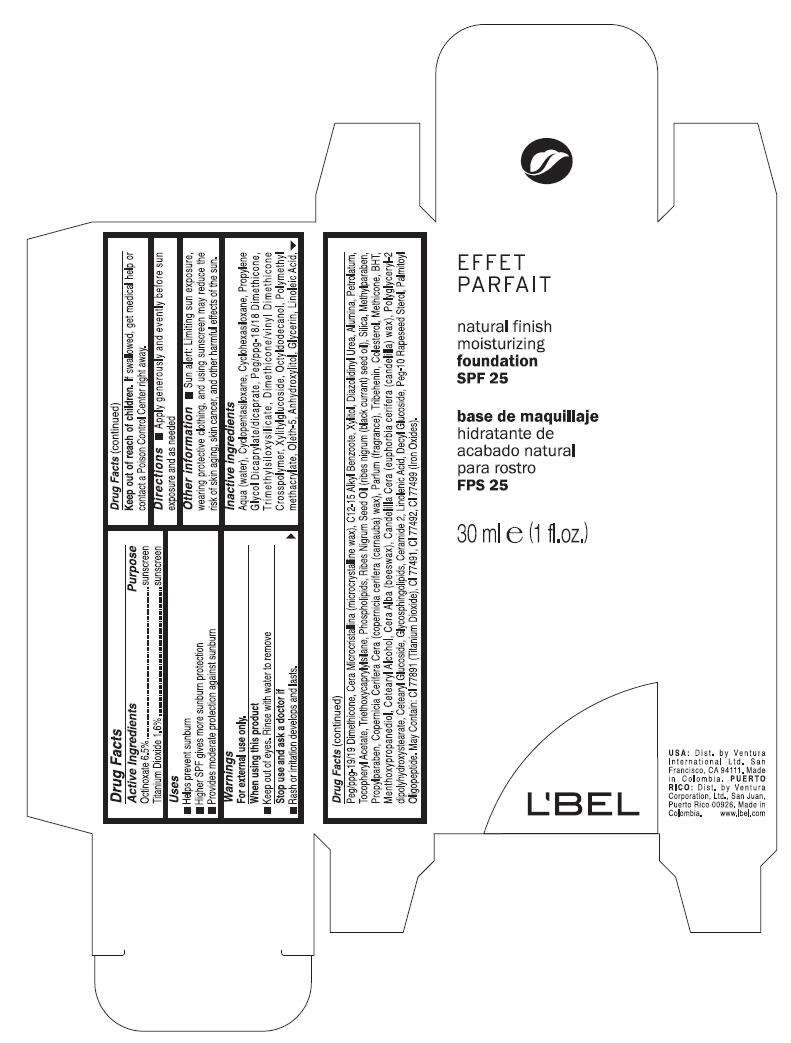
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active Ingredient | Purpose |
| Octinoxate 6.5%, | Sunscreen |
| Titanium Dioxide 1.6% | Sunscreen |
EFFET PARFAIT Uses
- Helps prevent sunburn
- Higher SPF gives more sunburn protection
- Provides moderate protection against sunburn
Warnings
For external use only
When using this product
- Keep out of eyes. Rinse with water to remove
Stop use and ask a doctor if
- Rash or irritation develops and lasts
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply generously and evently before sun exposure and as needed
EFFET PARFAIT Other information
- Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreen may reduce the risk of skin aging, skin cancer, and other harmful effects of the sun.
Inactive ingredients
Aqua(water),Cyclopentasiloxane, Cyclohexasiloxane, Propylene Glycol Dicaprylate/dicaprate, Peg/ppg-18/18 Dimethicone, Trimethylsiloxysilicate , Dimethicone/vinyl Dimethicone Crosspolymer, Xylitylglucoside, Octyldodecanol, Polymethyl methacrylate, Oleth-5, Anhydroxylitol, Glycerin, Linoleic Acid, Peg/ppg-19/19 Dimethicone, Cera Microcristallina (microcrystalline wax), C12-15 Alkyl Benzoote, Xylitol, Diazolidinyl Urea, Alumina, Petrolatum, Tocopheryl Acetate, Triethoxycaprylylsilane, Phospholipids, Ribes Nigrum Seed Oil (ribes nigrum (black currant) seed oil), Silica, Methylparaben, Propylparaben, Copernicia Cerifera Cera (copernicia cerifera (carnauba) wax), Parfum (fragrance), Tribehenin, Colesterol, Methicone, BHT, Menthoxypropanediol, Cetearyl Alcohol, Cera Alba (beeswax), Candelilla Cera (euphorbia cerifera (candelilla) wax), Polyglyceryl-2 dipolyhydroxystearate, Cetearyl Glucoside, Glycosphingolipids, Ceramide 2, Linolenic Acid, Decyl Glucoside, Peg-10 Rapeseed Sterol, Palmitoyl Oligopeptide. May Contain:CI 77891 (Titanium Dioxide), CI 77491, CI 77492, CI 77499 (Iron Oxides)
PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton
EFFET
PARFAIT
natural finish
moisturizing
foundation
SPF 25
30 ml e (1 fl.oz.)
L'BEL
EFFET PARFAITOctinoxate and Titanium Dioxide LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||