Enalapril Maleate
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- ENALAPRIL MALEATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS AND METABOLISM
- PHARMACODYNAMICS AND CLINICAL EFFECTS
- INDICATIONS & USAGE
- ENALAPRIL MALEATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- ENALAPRIL MALEATE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
USE IN PREGNANCYWhen used in pregnancy during the second and third trimesters, ACE inhibitors can cause injury and even death to the developing fetus.
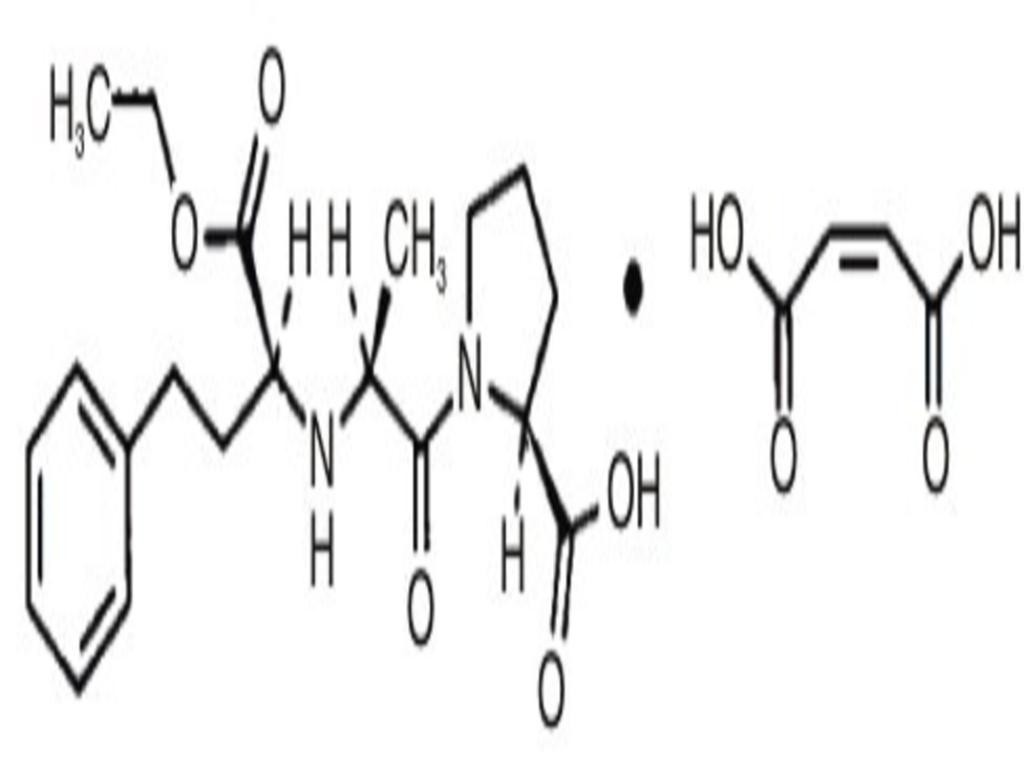
ENALAPRIL MALEATE DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanism of ActionPHARMACOKINETICS AND METABOLISM
PHARMACODYNAMICS AND CLINICAL EFFECTS
SURVIVAL (%)
INDICATIONS & USAGE
Hypertension
Heart Failure
Asymptomatic Left Ventricular Dysfunction
ENALAPRIL MALEATE CONTRAINDICATIONS
WARNINGS
Anaphylactoid and Possibly Related ReactionsHead and Neck Angioedema:Where there is involvement of the tongue, glottis or larynx, likely to cause airway obstruction, appropriate therapy, e.g., Subcutaneous epinephrine solution 1:1000 (0.3 mL to 0.5 mL) and/or measures necessary to ensure a patent airway, should be promptly provided.
Hypotension
Neutropenia/Agranulocytosis
Hepatic Failure
Fetal/Neonatal Morbidity and Mortality
PRECAUTIONS
General
Evaluation of patients with hypertension or heart failure should always include assessment of renal function.
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
ENALAPRIL MALEATE ADVERSE REACTIONS
HYPERTENSION
HEART FAILURE
Clinical Laboratory Test Findings
OVERDOSAGE
DOSAGE & ADMINISTRATION
HypertensionHOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Enalapril MaleateEnalapril Maleate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!