Enalapril Maleate
FULL PRESCRIBING INFORMATION: CONTENTS*
- USE IN PREGNANCY
- ENALAPRIL MALEATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- ENALAPRIL MALEATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ENALAPRIL MALEATE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
USE IN PREGNANCY
When used in pregnancy during the second and third trimesters, ACE inhibitors can cause injury and even death to the developing fetus.When pregnancy is detected, enalapril maleate tablets should be discontinued as soon as possible. (SeeWARNINGS: Fetal/Neonatal Morbidity and Mortality.)ENALAPRIL MALEATE DESCRIPTION
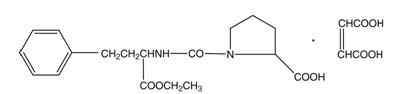
C20H28N2O5M.W. 492.53
CLINICAL PHARMACOLOGY
Mechanism of ActionPRECAUTIONS.) Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity.
Pharmacokinetics and Metabolism
DOSAGE AND ADMINISTRATION.) Enalaprilat is dialyzable at the rate of 62 mL/min.
Studies in dogs indicate that enalapril crosses the blood-brain barrier poorly, if at all; enalaprilat does not enter the brain. Multiple doses of enalapril maleate in rats do not result in accumulation in any tissues. Milk of lactating rats contains radioactivity following administration of 14C-enalappril maleate. Radioactivity was found to cross the placenta following administration of labeled drug to pregnant hamsters.
Pharmacodynamics and Clinical Effects
WARNINGS.)
DOSAGE AND ADMINISTRATION).
PRECAUTIONS: Drug Interactions.)
Six Months One Year
Clinical Pharmacology in Pediatric Patients
DOSAGE AND ADMINISTRATION: Pediatric Hypertensive Patients: Preparation of Suspension).
INDICATIONS & USAGE
HypertensionHeart Failure
CLINICAL PHARMACOLOGY: Heart Failure, Mortality Trialsfor details and limitations of survival trials).
Asymptomatic Left Ventricular Dysfunction
CLINICAL PHARMACOLOGY: Heart Failure, Mortality Trials for details and limitations of survival trials).
WARNINGS.)
WARNINGS: Head and Neck Angioedema.)
ENALAPRIL MALEATE CONTRAINDICATIONS
WARNINGS
Anaphylactoid and Possibly Related ReactionsWhere there is involvement of the tongue, glottis or larynx, likely to cause airway obstruction, appropriate therapy, e.g., subcutaneous epinephrine solution 1:1000 (0.3 mL to 0.5 mL) and/or measures necessary to ensure a patent airway, should be promptly provided.(SeeADVERSE REACTIONS.)
INDICATIONS AND USAGEandCONTRAINDICATIONS).
Hypotension
DOSAGE AND ADMINISTRATION.) Patients at risk for excessive hypotension, sometimes associated with oliguria and/or progressive azotemia, and rarely with acute renal failure and/or death, include those with the following conditions or characteristics: heart failure, hyponatremia, high dose diuretic therapy, recent intensive diuresis or increase in diuretic dose, renal dialysis, or severe volume and/or salt depletion of any etiology. It may be advisable to eliminate the diuretic (except in patients with heart failure), reduce the diuretic dose or increase salt intake cautiously before initiating therapy with enalapril in patients at risk for excessive hypotension who are able to tolerate such adjustments. (SeePRECAUTIONS: Drug InteractionsandADVERSE REACTIONS.) In patients at risk for excessive hypotension, therapy should be started under very close medical supervision and such patients should be followed closely for the first 2 weeks of treatment and whenever the dose of enalapril and/or diuretic is increased. Similar considerations may apply to patients with ischemic heart or cerebrovascular disease, in whom an excessive fall in blood pressure could result in a myocardial infarction or cerebrovascular accident.
Neutropenia/Agranulocytosis
Hepatic Failure
Fetal/Neonatal Morbidity and Mortality
PRECAUTIONS
GeneralEvaluation of patients with hypertension or heart failure should always include assessment of renal function. (See DOSAGE AND ADMINISTRATION.)
Drug Interactions.)
Information for Patients
Drug Interactions
WARNINGSandDOSAGE AND ADMINISTRATION.)
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
WARNINGS: Fetal/Neonatal Morbidity an Mortality.
Nursing Mothers
Pediatric Use
CLINICAL PHARMACOLOGY: Clinical Pharmacology in Pediatric PatientsandDOSAGE AND ADMINISTRATION).
ENALAPRIL MALEATE ADVERSE REACTIONS
Hypertension
Adverse experiences occurring in greater than 1% of patients with hypertension treated with enalapril in controlled clinical trials shown below. In patients treated with enalapril, the maximum duration of therapy was 3 years; in placebo treated patients the maximum duration of therapy was 12 weeks.
Enalapril Maleate
(n = 2,314)
Incidence
(discontinuation) Placebo
(n = 230)
Incidence
Heart Failure
Enalapril Maleate
(n = 673)
Incidence
(discontinuation) Placebo
(n = 339)
Incidence
Body as a Whole:Anaphylactoid reactions (seeWARNINGS: Anaphylactoid and Possibly Related Reactions).
Cardiovascular:WARNINGS: Hypotension
Digestive:Ileus, pancreatitis, hepatic failure, hepatitis (hepatocellular [proven on rechallenge] or cholestatic jaundice) (seeWARNINGS: Hepatic Failure), melena, anorexia, dyspepsia, constipation, glossitis, stomatitis, dry mouth.
Hematologic:Rare cases of neutropenia, thrombocytopenia and bone marrow depression.
Musculoskeletal:Muscle cramps.
Nervous/Psychiatric:Depression, confusion, ataxia, somnolence, insomnia, nervousness, peripheral neuropathy (e.g., paresthesia, dysesthesia), dream abnormality.
Respiratory:Bronchospasm, rhinorrhea, sore throat and hoarseness, asthma, upper respiratory infection, pulmonary infiltrates, eosinophilic pneumonitis.
Skin:Exfoliative dermatitis, toxic epidermal necrolysis, Stevens-Johnson Syndrome, pemphigus, herpes zoster, erythema multiforme, urticaria, pruritus, alopecia, flushing, diaphoresis, photosensitivity.
Special Senses:Blurred vision, taste alteration, anosmia, tinnitus, conjunctivitis, dry eyes, tearing.
Urogenital:Renal failure, oliguria, renal dysfunction (seePRECAUTIONSandDOSAGE AND ADMINISTRATION), flank pain, gynecomastia, impotence.
Miscellaneous:A symptom complex has been reported which may include some or all of the following: a positive ANA, an elevated erythrocyte sedimentation rate, arthralgia/arthritis, myalgia/myositis, fever, serositis, vasculitis, leukocytosis, eosinophilia, photosensitivity, rash and other dermatologic manifestations.
Angioedema:Angioedema has been reported in patients receiving enalapril, with an incidence higher in black than in nonblack patients. Angioedema associated with laryngeal edema may be fatal. If angioedema of the face, extremities, lips, tongue, glottis and/or larynx occurs, treatment with enalapril should be discontinued and appropriate therapy instituted immediately. (SeeWARNINGS.)
Hypotension:In the hypertensive patients, hypotension occurred in 0.9% and syncope occurred in 0.5% of patients following the initial dose or during extended therapy. Hypotension or syncope was a cause for discontinuation of therapy in 0.1% of hypertensive patients. In heart failure patients, hypotension occurred in 6.7% and syncope occurred in 2.2% of patients. Hypotension or syncope was a cause for discontinuation of therapy in 1.9% of patients with heart failure. (SeeWARNINGS.)
Fetal/Neonatal Morbidity and Mortality:SeeWARNINGS: Fetal/Neonatal Morbidity and Mortality.
Cough:SeePRECAUTIONS: Cough.
Pediatric Patients
Clinical Laboratory Test Findings
PRECAUTIONS), hyponatremia.
PRECAUTIONS.) In patients with heart failure who were also receiving diuretics with or without digitalis, increases in blood urea nitrogen or serum creatinine, usually reversible upon discontinuation of enalapril and/or other concomitant diuretic therapy, were observed in about 11% of patients. Increases in blood urea nitrogen or creatinine were a cause for discontinuation in 1.2% of patients.
WARNINGS: Hepatic Failure).
OVERDOSAGE
WARNINGS: Anaphylactoid Reactions During Membrane Exposure.)
DOSAGE & ADMINISTRATION
HypertensionWARNINGS.) If the patient's blood pressure is not controlled with enalapril maleate tablets alone, diuretic therapy may be resumed.
WARNINGSandPRECAUTIONS: Drug Interactions.)
PRECAUTIONS).
Dosage Adjustment in Hypertensive Patients with Renal Impairment
*
WARNINGS: Anaphylactoid Reactions During Membrane Exposure.
Renal Status Creatinine-Clearance
mL/min Initial Dose
mg/day *
Heart Failure
WARNINGSandPRECAUTIONS: Drug Interactions.) If possible, the dose of any concomitant diuretic should be reduced which may diminish the likelihood of hypotension. The appearance of hypotension after the initial dose of enalapril maleate tablets does not preclude subsequent careful dose titration with the drug, following effective management of the hypotension.
Asymptomatic Left Ventricular Dysfunction
WARNINGSandPRECAUTIONS: Drug Interactions.) If possible, the dose of any concomitant diuretic should be reduced which may diminish the likelihood of hypotension. The appearance of hypotension after the initial dose of enalapril maleate tablets does not preclude subsequent careful dose titration with the drug, following effective management of the hypotension.
Dosage Adjustment in Patients with Heart Failure and Renal Impairment or Hyponatremia
DOSAGE AND ADMINISTRATION: Heart Failure,WARNINGSandPRECAUTIONS: Drug Interactions.) The dose may be increased to 2.5 mg b.i.d., then 5 mg b.i.d. and higher as needed, usually at intervals of 4 days or more if at the time of dosage adjustment there is not excessive hypotension or significant deterioration of renal function. The maximum daily dose is 40 mg.
Pediatric Hypertensive Patients
CLINICAL PHARMACOLOGY: Clinical Pharmacology in Pediatric Patients).
1to a polyethylene terephthalate (PET) bottle containing ten 20 mg tablets of enalapril maleate and shake for at least 2 minutes. Let concentrate stand for 60 minutes. Following the 60 minute hold time, shake the concentrate for an additional minute. Add 150 mL of Ora-Sweet SF2to the concentrate in the PET bottle and shake the suspension to disperse the ingredients. The suspension should be refrigerated at 2to 8(36to 46and can be stored for up to 30 days. Shake the suspension before each use.
1
2
HOW SUPPLIED
M overE15 on one side and scored on the other side. They are available as follows:
M overE16 on one side and scored on the other side. They are available as follows:
M overE17 on one side and blank on the other side. They are available as follows:
M overE18 on one side and blank on the other side. They are available as follows:
STORAGE AND HANDLING
Store at 20 to 25 C (68 to 77 F).[See USP Controlled Room Temperature.]PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Enalapril MaleateENALAPRIL MALEATE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!