Enalapril Maleate
FULL PRESCRIBING INFORMATION: CONTENTS*
- ENALAPRIL MALEATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- ENALAPRIL MALEATE INDICATIONS AND USAGE
- ENALAPRIL MALEATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ENALAPRIL MALEATE ADVERSE REACTIONS
- OVERDOSAGE
- ENALAPRIL MALEATE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Product Information
ENALAPRIL MALEATE TABLETS,
USP
Rx only
USE IN PREGNANCY
When used in pregnancy during the
second and third trimesters, ACE inhibitors can cause injury and even death to
the developing fetus. When pregnancy is detected, enalapril maleate
should be discontinued as soon as possible. See WARNINGS, Fetal / Neonatal Morbidity and Mortality.
ENALAPRIL MALEATE DESCRIPTION
Enalapril maleate is the maleate salt of enalapril, the ethyl ester of a
long-acting angiotensin converting enzyme inhibitor, enalaprilat. Enalapril
maleate is chemically described as
L-Proline,1-[N-[1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanyl]- , (S)-,
(Z)-2-butenedioate (1:1). Its molecular formula is, C20H28N2O5·C4H4O4, and its structural formula is:
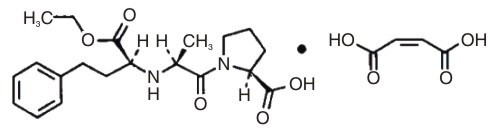
Enalapril maleate is a white to off-white, crystalline powder with a molecular
weight of 492.53. It is sparingly soluble in water, soluble in ethanol, and
freely soluble in methanol.
Enalapril is a pro-drug; following oral
administration, it is bioactivated by hydrolysis of the ethyl ester to
enalaprilat, which is the active angiotensin converting enzyme
inhibitor.
Enalapril maleate is supplied as 2.5 mg, 5 mg,10 mg and 20 mg
tablets for oral administration. In addition, each tablet contains the following
inactive ingredients: hypromellose, anhydrous lactose, corn starch, stearic acid
and talc. The 10 mg and 20 mg tablets also contain iron oxides.
CLINICAL PHARMACOLOGY
14Hypertension:
Heart Failure:
Heart Failure, Mortality Trials:
|
|
SURVIVAL (%) | |
|---|---|---|
|
|
Six Months | One Year |
| Enalapril Maleate (n=127) |
74 |
64 |
| Placebo (n=126) |
56 |
48 |
Clinical Pharmacology in Pediatric Patients
Preparation of Suspension
ENALAPRIL MALEATE INDICATIONS AND USAGE
Heart Failure, Mortality TrialsHeart Failure, Mortality Trials
Head and Neck Angioedema
ENALAPRIL MALEATE CONTRAINDICATIONS
Enalapril maleate is contraindicated in patients who are hypersensitive to this product and in patients with a history of angioedema related to previous treatment with an angiotensin converting enzyme inhibitor and in patients with hereditary or idiopathic angioedema.
WARNINGS
Head and Neck Angioedema: Where there is involvement of the tongue, glottis or larynx, likely to cause airway obstruction, appropriate therapy, e.g., Subcutaneous epinephrine solution 1:1000 (0.3 mL to 0.5 mL) and/or measures necessary to ensure a patent airway, should be promptly provided.
Intestinal Angioedema:
Anaphylactoid reactions during desensitization:
Anaphylactoid reactions during membrane exposure: Drug Interactions
in utero
PRECAUTIONS
Aortic Stenosis/Hypertrophic Cardiomyopathy:Impaired Renal Function:
Evaluation of patients with hypertension or heart failure should always include assessment of renal function.See
Hyperkalemia:
Drug Interactions
Cough:
Surgery/Anesthesia:Angioedema:
Hypotension:
Hyperkalemia:
Neutropenia:
Pregnancy:
Hypotension Patients on Diuretic Therapy:
Agents Causing Renin Release:
Non-steroidal Anti-inflammatory Agents:
Other Cardiovascular Agents:
Agents Increasing Serum Potassium:
Lithium:
Gold:
E.coli,
Pregnancy Categories Cand DFetal/Neonatal Morbidity and Mortality.Clinical Pharmacology in Pediatric Patients
2
ENALAPRIL MALEATE ADVERSE REACTIONS
|
|
Enalapril Maleate (n=2314) Incidence (discontinuation) |
Placebo (n=230) Incidence |
| Body As A Whole |
|
|
| Fatigue |
3.0 (less than 0.1) |
2.6 |
| Orthostatic Effets |
1.2 (less than 0.1) | 0.0 |
| Asthenia |
1.1 (0.1) | 0.9 |
| Digestive |
|
|
| Diarrhea |
1.4 (less than 0.1) | 1.7 |
| Nausea |
1.4 (0.2) | 1.7 |
| Nervous/Psychiatric |
|
|
| Headache |
5.2 (0.3) | 9.1 |
| Dizziness |
4.3 (0.4) | 4.3 |
| Respiratory |
|
|
| Cough |
1.3 (0.1) | 0.9 |
| Skin |
|
|
| Rash |
1.4 (0.4) | 0.4 |
|
|
Enalapril Maleate (n=673) Incidence (discontinuation) |
Placebo (n=339) Incidence |
| Body As A Whole |
|
|
| Orthostatic Effects | 2.2 (0.1) | 0.3 |
| Syncope | 2.2 (0.1) | 0.9 |
| Chest Pain | 2.1 (0.0) |
2.1 |
| Fatigue | 1.8 (0.0) | 1.8 |
| Abdominal Pain | 1.6 (0.4) | 2.1 |
| Asthenia | 1.6 (0.1) |
0.3 |
| Cardiovascular |
|
|
| Hypotension | 6.7 (1.9) |
0.6 |
| Orthostatic Hypotension | 1.6 (0.1) |
0.3 |
| Angina Pectoris | 1.5 (0.1) |
1.8 |
| Myocardial Infarction | 1.2 (0.3) |
1.8 |
| Digestive |
|
|
| Diarrhea | 2.1 (0.1) | 1.2 |
| Nausea | 1.3 (0.1) |
0.6 |
| Vomiting | 1.3 (0.0) |
0.9 |
| Nervous/Psychiatric |
|
|
| Dizziness | 7.9(0.6) | 0.6 |
| Headache | 1.8 (0.1) | 0.9 |
| Vertigo | 1.6 (0.1) |
1.2 |
| Respiratory |
|
|
| Cough | 2.2 (0.0) | 0.6 |
| Bronchitis | 1.3 (0.0) | 0.9 |
| Dyspnea | 1.3 (0.1) | 0.4 |
| Pneumonia | 1.0 (0.0) | 2.4 |
| Skin |
|
|
| Rash | 1.3 (0.0) |
2.4 |
| Urogenital |
|
|
| Urinary Tract Infection | 1.3 (0.0) | 2.4 |
Body As A Whole: Anaphylactoid and Possibly Related Reactions
Cardiovascular: Hypotension
Digestive:Hepatic Failure
Hematologic:
Musculoskeletal
Nervous/Psychiatric:
Respiratory:
Skin:
Special Senses:
Urogenital:
Miscellaneous:
Angioedema:
Hypotension:
Fetal/Neonatal Morbidity and MortalityFetal/Neonatal Morbidity and Mortality
CoughCough.
Pediatric Patients
Serum Electrolytes:
Creatinine, Blood Urea Nitrogen:
Hematology:
Liver Function Tests:Hepatic Failure
OVERDOSAGE
Limited data are available in regard to overdosage in humans. Single oral doses
of enalapril above 1,000 mg/kg and ≥1,775 mg/kg were associated with lethality
in mice and rats, respectively.
The most likely manifestation of
overdosage would be hypotension, for which the usual treatment would be
intravenous infusion of normal saline solution.
Enalaprilat may be
removed from general circulation by hemodialysis and has been removed from
neonatal circulation by peritoneal dialysis. (See WARNINGS, Anaphylactoid reactions during membrane exposure.)
ENALAPRIL MALEATE DOSAGE AND ADMINISTRATION
Drug Interactions
| Renal Status |
Creatinine- Clearance ml/min |
Initial Dose mg/day |
| Normal Renal Function |
>80 mL/min |
5 mg |
| Mild Impairment |
≤80> 30 mL/min | 5 mg |
| Moderate to Severe Impairment |
≤30 mL/min | 2.5 mg |
| Dialysis Patients*** |
- - |
2.5 mg on dialysis days†
|
†
Heart Failure
Drug Interactions
Asymptomatic Left Ventricular Dysfunction
Drug Interactions
Dosage Adjustment in Patients with Heart Failure and Renal Impairment or Hyponatremia
Heart Failure, Drug Interactions
Pediatric Hypertensive Patients
Clinical Pharmacology in Pediatric Patients.
2
Preparation of Suspension (for 200 mL of a 1.0 mg/mL suspension)
®**TM***
HOW SUPPLIED
|
Quantity
|
NDC Number
|
Strength
|
Description
|
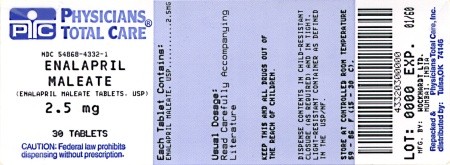
| Bottles of 30 Bottles of 60 Bottles of 100 |
NDC 54868-4332-1 NDC 54868-4332-0 NDC 54868-4332-2 |
2.5 mg |
White, rount flat-faced beveled edged, compressed tablets with W on one side and breakline on the other side. |
| Bottles of 30 Bottles of 60 Bottles of 90 Bottles of 100 |
NDC 54868-4357-0 NDC 54868-4357-1 NDC 54868-4357-3 NDC 54868-4357-2 |
5 mg |
White, rount flat-faced beveled edged, compressed tablets with W on one side
and breakline on the other
side. |
| Bottles of 30 Bottles of 60 Bottles of 90 Bottles of 100 |
NDC 54868-4358-0 NDC 54868-4358-1 NDC 54868-4358-3 NDC 54868-4358-2 |
10 mg |
Light Salmon, round flat-faced beveled edged, compressed tablets with W on one side plain on the other side. |
| Bottles of 30 Bottles of 60 Bottles of 90 Bottles of 100 |
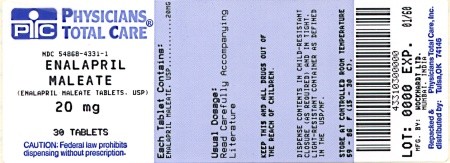
NDC 54868-4331-1 NDC 54868-4331-2 NDC 54868-4331-3 NDC 54868-4331-0 |
20 mg |
Light Beige, rount flat-faced beveled edged, compressed tablets with W on one side plain on the other side. |
S
to
rage
Store below 30°C
(86°F) and avoid transient temperatures above 50°C (122°F). Keep container
tightly closed. Protect from moisture.
Dispense in a tight container as
per USP, if product package is
subdivided.
___________________________________________________________________________________________________________________________________
** Registered trademark of Alza Corporation.
*** Trademark of Paddock Laboratories, Inc.
Manufactured
by:
Wockhardt Limited,
Mumbai,
India.
Distributed by:
Wockhardt USA LLC.
20 Waterview Blvd.
Parsippany, NJ 07054
USA.
Rev.221209
Relabeling and Repackaging by:
Physicians Total Care, Inc.
Tulsa, OK 74146
PRINCIPAL DISPLAY PANEL
Enalapril Maleate Tablets
2.5 mg
5 mg

10 mg

20 mg
Enalapril MaleateEnalapril Maleate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enalapril MaleateEnalapril Maleate TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enalapril MaleateEnalapril Maleate TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enalapril MaleateEnalapril Maleate TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||