EPINASTINE HYDROCHLORIDE
Sun Pharmaceutical Industries Limited
Epinastine Hydrochloride Ophthalmic Solution 0.05%Sterile
FULL PRESCRIBING INFORMATION: CONTENTS*
- EPINASTINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- EPINASTINE HYDROCHLORIDE INDICATIONS AND USAGE
- EPINASTINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- EPINASTINE HYDROCHLORIDE ADVERSE REACTIONS
- EPINASTINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON
FULL PRESCRIBING INFORMATION
EPINASTINE HYDROCHLORIDE DESCRIPTION
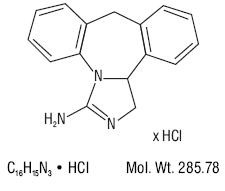
Chemical Name:
Each mL contains: ActivePreservative: Inactives:
CLINICAL PHARMACOLOGY
112122
Clinical studies:
EPINASTINE HYDROCHLORIDE INDICATIONS AND USAGE
EPINASTINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
Information for Patients:
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Salmonella in vitro in vitro in vivo in vivo/in vitro
Pregnancy:
Teratogenic Effects: Pregnancy Category C
Nursing Mothers:
Pediatric Use:
Geriatric Use:
EPINASTINE HYDROCHLORIDE ADVERSE REACTIONS
EPINASTINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
HOW SUPPLIED
Storage:
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Industries Ltd.
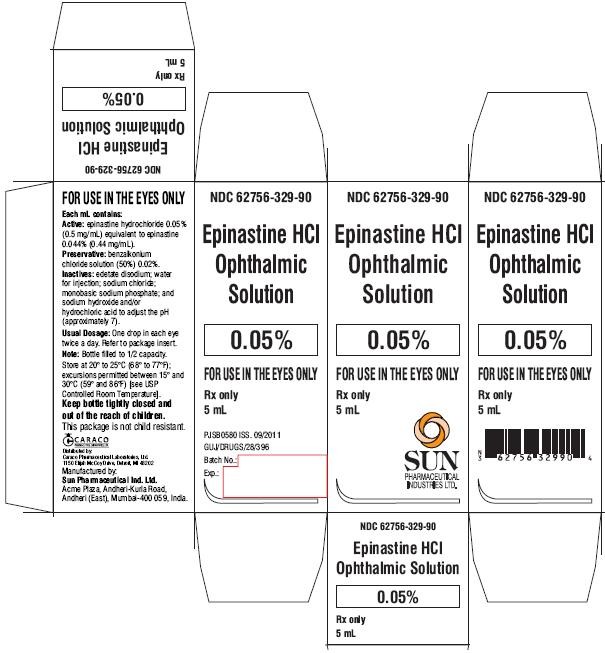
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON
NDC 62756-329-90
Epinastine HCl Ophthalmic Solution
0.05%
FOR USE IN THE EYES ONLY
Rx only
5 mL
SUN PHARMACEUTICAL INDUSTRIES LTD.
EPINASTINE HYDROCHLORIDEEPINASTINE HYDROCHLORIDE SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!