Epirubicin Hydrochloride
Epirubicin Hydrochloride for Injection
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING
- EPIRUBICIN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- EPIRUBICIN HYDROCHLORIDE INDICATIONS AND USAGE
- EPIRUBICIN HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- EPIRUBICIN HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- EPIRUBICIN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- 483478
- 481517
FULL PRESCRIBING INFORMATION
Rx only
WARNING
-
Severe local tissue necrosis will occur if there is extravasation during administration (See PRECAUTIONS ). Epirubicin must not be given by the intramuscular or subcutaneous route.
-
Myocardial toxicity, manifested in its most severe form by potentially fatal congestive heart failure (CHF), may occur either during therapy with epirubicin or months to years after termination of therapy. The probability of developing clinically evident CHF is estimated as approximately 0.9% at a cumulative dose of 550 mg/m2, 1.6% at 700 mg/m2, and 3.3% at 900 mg/m2. In the adjuvant treatment of breast cancer, the maximum cumulative dose used in clinical trials was 720 mg/m2. The risk of developing CHF increases rapidly with increasing total cumulative doses of epirubicin in excess of 900 mg/m2; this cumulative dose should only be exceeded with extreme caution. Active or dormant cardiovascular disease, prior or concomitant radiotherapy to the mediastinal/pericardial area, previous therapy with other anthracyclines or anthracenediones, or concomitant use of other cardiotoxic drugs may increase the risk of cardiac toxicity. Cardiac toxicity with Epirubicin Hydrochloride for Injection may occur at lower cumulative doses whether or not cardiac risk factors are present.
-
Secondary acute myelogenous leukemia (AML) has been reported in patients with breast cancer treated with anthracyclines,including epirubicin. The occurrence of refractory secondary leukemia is more common when such drugs are given in combination with DNA-damaging antineoplastic agents, when patients have been heavily pretreated with cytotoxic drugs, or when doses of anthracyclines have been escalated. The cumulative risk of developing treatment-related AML or myodysplastic syndrome (MDS), in 7110 patients with breast cancer who received adjuvant treatment with epirubicin-containing regimens, was estimated as 0.27% at 3 years, 0.46% at 5 years, and 0.55% at 8 years.
-
Dosage should be reduced in patients with impaired hepatic function (see DOSAGE AND ADMINISTRATION ).
-
Severe myelosuppression may occur.
-
Epirubicin should be administered only under the supervision of a physician who is experienced in the use of cancer chemotherapeutic agents.
EPIRUBICIN HYDROCHLORIDE DESCRIPTION
Epirubicin Hydrochloride for Injection is an anthracycline cytotoxic agent, intended for intravenous administration. Epirubicin Hydrochloride for Injection is supplied as a sterile, orange-red, lyophilized powder in single-dose vials containing 50 mg or 200 mg of epirubicin hydrochloride. Each 50 mg and 200 mg vial contains 250 mg and 1000 mg inactive ingredient, lactose, respectively.
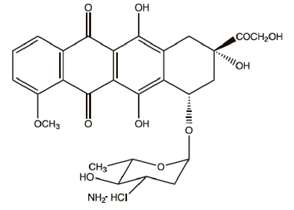
Epirubicin hydrochloride is the 4-epimer of doxorubicin and is a semi-synthetic derivative of daunorubicin. The chemical name is (8S- cis )-10-[(3-amino-2,3,6-trideoxy-α-L- arabino -hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-5,12-naphthacenedione hydrochloride. The active ingredient is a red-orange hygroscopic powder, with the empirical formula C27H29NO11HCl and a molecular weight of 579.95. The structural formula is as follows:
CLINICAL PHARMACOLOGY
Epirubicin is an anthracycline cytotoxic agent. Although it is known that anthracyclines can interfere with a number of biochemical and biological functions within eukaryotic cells, the precise mechanisms of epirubicin’s cytotoxic and/or antiproliferative properties have not been completely elucidated.
Epirubicin forms a complex with DNA by intercalation of its planar rings between nucleotide base pairs, with consequent inhibition of nucleic acid (DNA and RNA) and protein synthesis. Such intercalation triggers DNA cleavage by topoisomerase II, resulting in cytocidal activity. Epirubicin also inhibits DNA helicase activity, preventing the enzymatic separation of double-stranded DNA and interfering with replication and transcription. Epirubicin is also involved in oxidation/reduction reactions by generating cytotoxic free radicals. The antiproliferative and cytotoxic activity of epirubicin is thought to result from these or other possible mechanisms.
Epirubicin is cytotoxic in vitro to a variety of established murine and human cell lines and primary cultures of human tumors. It is also active in vivo against a variety of murine tumors and human xenografts in athymic mice, including breast tumors.
Pharmacokinetics
Epirubicin pharmacokinetics are linear over the dose range of 60 to 150 mg/m2 and plasma clearance is not affected by the duration of infusion or administration schedule. Pharmacokinetic parameters for epirubicin following 6- to 10-minute, single-dose intravenous infusions of epirubicin at doses of 60 to 150 mg/m2 in patients with solid tumors are shown in Table 1. The plasma concentration declined in a triphasic manner with mean half-lives for the alpha, beta, and gamma phases of about 3 minutes, 2.5 hours, and 33 hours, respectively.
|
Dose2
(mg/m2) |
Cmax
3
(μg/mL) |
AUC 4
(μg • h/mL) |
t1/2
5
(hours) |
CL6
(L/hour) |
Vss
7
(L/kg) |
|---|---|---|---|---|---|
|
60 |
5.7 ± 1.6 |
1.6 ± 0.2 |
35.3 ± 9 |
65 ± 8 |
21 ± 2 |
|
75 |
5.3 ± 1.5 |
1.7 ± 0.3 |
32.1 ± 5 |
83 ± 14 |
27 ± 11 |
|
120 |
9.0 ± 3.5 |
3.4 ± 0.7 |
33.7 ± 4 |
65 ± 13 |
23 ± 7 |
|
150 |
9.3 ± 2.9 |
4.2 ± 0.8 |
31.1 ± 6 |
69 ± 13 |
21 ± 7 |
|
1Advanced solid tumor cancers, primarily of the lung 2N=6 patients per dose level 3Plasma concentration at the end of 6 to 10 minute infusion 4Area under the plasma concentration curve 5Half-life of terminal phase 6Plasma clearance 7Steady state volume of distribution |
|||||
Distribution
Following intravenous administration, epirubicin is rapidly and widely distributed into the tissues. Binding of epirubicin to plasma proteins, predominantly albumin, is about 77% and is not affected by drug concentration. Epirubicin also appears to concentrate in red blood cells; whole blood concentrations are approximately twice those of plasma.
Metabolism
Epirubicin is extensively and rapidly metabolized by the liver and is also metabolized by other organs and cells, including red blood cells. Four main metabolic routes have been identified:
(1) reduction of the C-13 keto-group with the formation of the 13(S)-dihydro derivative, epirubicinol; (2) conjugation of both the unchanged drug and epirubicinol with glucuronic acid; (3) loss of the amino sugar moiety through a hydrolytic process with the formation of the doxorubicin and doxorubicinol aglycones; and (4) loss of the amino sugar moiety through a redox process with the formation of the 7-deoxy-doxorubicin aglycone and 7-deoxydoxorubicinol aglycone. Epirubicinol has in vitro cytotoxic activity one-tenth that of epirubicin. As plasma levels of epirubicinol are lower than those of the unchanged drug, they are unlikely to reach in vivo concentrations sufficient for cytotoxicity. No significant activity or toxicity has been reported for the other metabolites.
Excretion
Epirubicin and its major metabolites are eliminated through biliary excretion and, to a lesser extent, by urinary excretion. Mass-balance data from 1 patient found about 60% of the total radioactive dose in feces (34%) and urine (27%). These data are consistent with those from 3 patients with extrahepatic obstruction and percutaneous drainage, in whom approximately 35% and 20% of the administered dose were recovered as epirubicin or its major metabolites in bile and urine, respectively, in the 4 days after treatment.
Pharmacokinetics in Special Populations
Age
A population analysis of plasma data from 36 cancer patients (13 males and 23 females, 20 to 73 years) showed that age affects plasma clearance of epirubicin in female patients. The predicted plasma clearance for a female patient of 70 years of age was about 35% lower than that for a female patient of 25 years of age. An insufficient number of males > 50 years of age were included in the study to draw conclusions about age-related alterations in clearance in males. Although a lower epirubicin starting dose does not appear necessary in elderly female patients, and was not used in clinical trials, particular care should be taken in monitoring toxicity when epirubicin is administered to female patients > 70 years of age. (See PRECAUTIONS .)
Gender
In patients ≤ 50 years of age, mean clearance values in adult male and female patients were similar. The clearance of epirubicin is decreased in elderly women (see Pharmacokinetics in Special Populations, Age ).
Pediatric
The pharmacokinetics of epirubicin in pediatric patients have not been evaluated.
Race
The influence of race on the pharmacokinetics of epirubicin has not been evaluated.
Hepatic Impairment
Epirubicin is eliminated by both hepatic metabolism and biliary excretion and clearance is reduced in patients with hepatic dysfunction. In a study of the effect of hepatic dysfunction, patients with solid tumors were classified into 3 groups. Patients in Group 1 (n=22) had serum AST (SGOT) levels above the upper limit of normal (median: 93 IU/L) and normal serum bilirubin levels (median: 0.5 mg/dL) and were given epirubicin doses of 12.5 to 90 mg/m2. Patients in Group 2 had alterations in both serum AST (median: 175 IU/L) and bilirubin levels (median: 2.7 mg/dL) and were treated with an epirubicin dose of 25 mg/m2 (n=8). Their pharmacokinetics were compared to those of patients with normal serum AST and bilirubin values, who received epirubicin doses of 12.5 to 120 mg/m2. The median plasma clearance of epirubicin was decreased compared to patients with normal hepatic function by about 30% in patients in Group 1 and by 50% in patients in Group 2. Patients with more severe hepatic impairment have not been evaluated. (See WARNINGS and DOSAGE AND ADMINISTRATION .)
Renal Impairment
No significant alterations in the pharmacokinetics of epirubicin or its major metabolite, epirubicinol, have been observed in patients with serum creatinine < 5 mg/dL. A 50% reduction in plasma clearance was reported in four patients with serum creatinine ≥ 5 mg/dL (see WARNINGS and DOSAGE AND ADMINISTRATION ). Patients on dialysis have not been studied.
Drug-Drug Interactions
Taxanes
Coadministration of paclitaxel or docetaxel did not affect the pharmacokinetics of epirubicin when given immediately following the taxane.
Cimetidine
Coadministration of cimetidine (400 mg twice daily for 7 days starting 5 days before chemotherapy) increased the mean AUC of epirubicin (100 mg/m2) by 50% and decreased its plasma clearance by 30% (see PRECAUTIONS ).
Drugs Metabolized by Cytochrome P-450 Enzymes
No systematic in vitro or in vivo evaluation has been performed to examine the potential for inhibition or induction by epirubicin of oxidative cytochrome P-450 isoenzymes.
CLINICAL STUDIES
Two randomized, open-label, multicenter studies evaluated the use of Epirubicin Hydrochloride for Injection 100 to 120 mg/m2 in combination with cyclophosphamide and fluorouracil for the adjuvant treatment of patients with axillary-node positive breast cancer and no evidence of distant metastatic disease (Stage II or III). Study MA-5 evaluated 120 mg/m2 of epirubicin per course in combination with cyclophosphamide and fluorouracil (CEF-120 regimen). This study randomized premenopausal and perimenopausal women with one or more positive lymph nodes to an epirubicin-containing CEF-120 regimen or to a CMF regimen. Study GFEA-05 evaluated the use of 100 mg/m2 of epirubicin per course in combination with fluorouracil and cyclophosphamide (FEC-100). This study randomized pre- and postmenopausal women to the FEC-100 regimen or to a lower-dose FEC-50 regimen. In the GFEA-05 study, eligible patients were either required to have ≥ 4 nodes involved with tumor or, if only 1 to 3 nodes were positive, to have negative estrogen- and progesterone-receptors and a histologic tumor grade of 2 or 3. A total of 1281 women participated in these studies. Patients with T4 tumors were not eligible for either study. Table 2 shows the treatment regimens that the patients received. The primary endpoint of the trials was relapse-free survival, i.e., time to occurrence of a local, regional, or distant recurrence, or disease-related death. Patients with contralateral breast cancer, second primary malignancy or death from causes other than breast cancer were censored at the time of the last visit prior to these events.
|
|
Treatment Groups |
Agent |
Regimen |
|
MA-51 |
CEF-120
(total, 6 cycles)2 |
Cyclophosphamide |
75 mg/m2 PO, d 1-14, q 28 days |
|
GFEA-053 |
FEC
-100 (total, 6 cycles) |
Fluorouracil |
600 mg/m2 IV, d 1, q 21 days |
|
1 In women who underwent lumpectomy, breast irradiation was to be administered after completion of study chemotherapy. |
|||
In the MA-5 trial, the median age of the study population was 45 years. Approximately 60% of patients had 1 to 3 involved nodes and approximately 40% had ≥ 4 nodes involved with tumor. In the GFEA-05 study, the median age was 51 years and approximately half of the patients were postmenopausal. About 17% of the study population had 1 to 3 positive nodes and 80% of patients had ≥ 4 involved lymph nodes. Demographic and tumor characteristics were well-balanced between treatment arms in each study.
The efficacy endpoints of relapse-free survival (RFS) and overall survival (OS) were analyzed using Kaplan-Meier methods in the intent-to-treat (ITT) patient populations in each study. Results for endpoints were initially analyzed after up to 5 years of follow-up and these results are presented in the text below and in Table 3. Results after up to 10 years of follow-up are presented in Table 3. In Study MA-5, epirubicin-containing combination therapy (CEF-120) showed significantly longer RFS than CMF (5-year estimates were 62% versus 53%; stratified log rank for the overall RFS p=0.013). The estimated reduction in risk of relapse was 24% at 5 years. The OS was also greater for the epirubicin-containing CEF-120 regimen than for the CMF regimen (5-year estimate 77% versus 70%; stratified log rank for overall survival p=0.043; non-stratified log rank p=0.13). The estimated reduction in the risk of death was 29% at 5 years.
In Study GFEA-05, patients treated with the higher-dose epirubicin regimen (FEC-100) had a significantly longer 5-year RFS (estimated 65% versus 52%, log rank for the overall RFS p=0.007) and OS (estimated 76% versus 65%, log rank for the overall survival p=0.007) than patients given the lower dose regimen (FEC-50). The estimated reduction in risk of relapse was 32%. The estimated reduction in the risk of death was 31% at 5 years. Results of follow-up up to 10 years (median follow-up = 8.8 years and 8.3 years, respectively for Study MA-5 and Study GFEA-05), are presented in Table 3.
Although the trials were not powered for subgroup analyses, in the MA-5 study improvements in favor of CEF-120 vs. CMF were observed, in RFS and OS both in patients with 1 to 3 node positive and in those with ≥ 4 node positive tumor involvement. In the GFEA-05 study improvements in RFS and OS were observed in both pre- and post-menopausal women treated with FEC-100 compared to FEC-50.
| MA-5 Study | GFEA-05 Study | |||
| CEF-120 N=356 |
CMF N=360 |
FEC-100 N=276 |
FEC-50 N=289 |
|
|
RFS at 5 yrs (%) |
62 |
53 |
65 |
52 |
|
Hazard ratio† |
0.76 |
0.68 |
||
|
2-sided 95% CI |
(0.60, 0.96) |
(0.52, 0.89) |
||
|
Log-rank Test stratified** |
(p = 0.013 |
(p = 0.007) |
||
|
OS at 5 yrs |
77 |
70 |
76 |
65 |
|
Hazard ratio† |
0.71 |
0.69 |
||
|
2-sided 95% CI |
(0.52, 0.98) |
(0.51, 0.92) |
||
|
Log-rank Test stratified** |
(p= 0.043) |
(p=0.007) |
||
|
RFS at 10 yrs (%) |
51 |
44 |
49 |
43 |
|
Hazard ratio† |
0.78 |
0.78 |
||
|
2-sided 95% CI |
(0.63, 0.96) |
(0.62, 0.99) |
||
|
Log-rank Test stratified** |
(p = 0.017) |
(p = 0.040) |
||
|
OS at 10 yrs (%) |
61 |
57 |
56 |
50 |
|
Hazard ratio† |
0.82 |
0.75 |
||
|
2-sided 95% CI |
(0.65, 1.04) |
(0.58, 0.96) |
||
|
Log-rank Test stratified** |
(p = 0.100) |
(p = 0.023) |
||
|
*Based on Kaplan-Meier estimates |
||||
The Kaplan-Meier curves for RFS and OS from Study MA-5 are shown in Figures 1 and 2 and those for study GFEA-05 are shown in Figures 3 and 4.
Figure 1. Relapse-Free Survival in Study MA-5
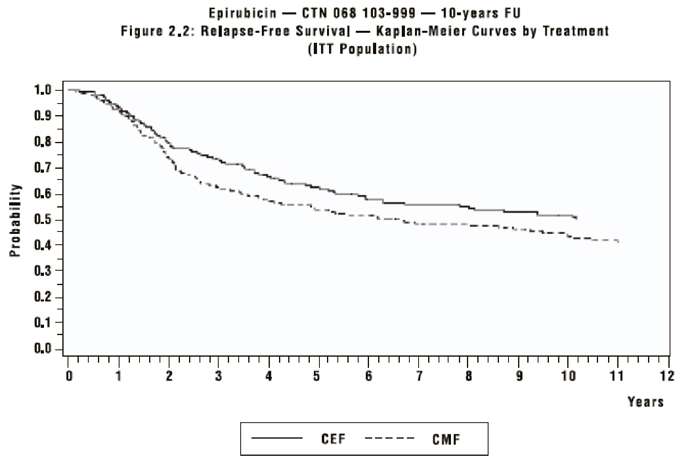
Figure 2. Overall Survival in Study MA-5

Figure 3. Relapse-Free Survival in Study GFEA-05
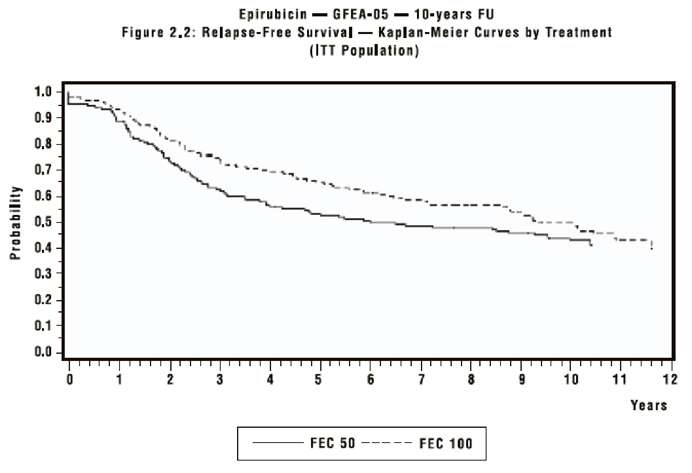
Figure 4. Overall Survival in Study GFEA-05

See Table 3 for statistics on 5 and 10 year analyses.
EPIRUBICIN HYDROCHLORIDE INDICATIONS AND USAGE
Epirubicin Hydrochloride for Injection is indicated as a component of adjuvant therapy in patients with evidence of axillary node tumor involvement following resection of primary breast cancer.
EPIRUBICIN HYDROCHLORIDE CONTRAINDICATIONS
Patients should not be treated with Epirubicin Hydrochloride for Injection if they have any of the following conditions: baseline neutrophil count <1500 cells/mm3; severe myocardial insufficiency, recent myocardial infarction, severe arrhythmias; previous treatment with anthracyclines up to the maximum cumulative dose; hypersensitivity to epirubicin, other anthracyclines, or anthracenediones; or severe hepatic dysfunction (see WARNINGS and DOSAGE AND ADMINISTRATION ).
WARNINGS
Epirubicin Hydrochloride for Injection should be administered only under the supervision of qualified physicians experienced in the use of cytotoxic therapy. Before beginning treatment with epirubicin, patients should recover from acute toxicities (such as stomatitis, neutropenia, thrombocytopenia, and generalized infections) of prior cytotoxic treatment. Also, initial treatment with Epirubicin Hydrochloride for Injection should be preceded by a careful baseline assessment of blood counts; serum levels of total bilirubin, AST, and creatinine; and cardiac function as measured by left ventricular ejection function (LVEF). Patients should be carefully monitored during treatment for possible clinical complications due to myelosuppression. Supportive care may be necessary for the treatment of severe neutropenia and severe infectious complications. Monitoring for potential cardiotoxicity is also important, especially with greater cumulative exposure to epirubicin.
Hematologic Toxicity
A dose-dependent, reversible leukopenia and/or neutropenia is the predominant manifestation of hematologic toxicity associated with epirubicin and represents the most common acute dose-limiting toxicity of this drug. In most cases, the white blood cell (WBC) nadir is reached 10 to 14 days from drug administration. Leukopenia/neutropenia is usually transient, with WBC and neutrophil counts generally returning to normal values by Day 21 after drug administration. As with other cytotoxic agents, Epirubicin Hydrochloride for Injection at the recommended dose in combination with cyclophosphamide and fluorouracil can produce severe leukopenia and neutropenia. Severe thrombocytopenia and anemia may also occur. Clinical consequences of severe myelosuppression include fever, infection, septicemia, septic shock, hemorrhage, tissue hypoxia, symptomatic anemia, or death. If myelosuppressive complications occur, appropriate supportive measures (e.g., intravenous antibiotics, colony-stimulating factors, transfusions) may be required. Myelosuppression requires careful monitoring. Total and differential WBC, red blood cell (RBC), and platelet counts should be assessed before and during each cycle of therapy with Epirubicin Hydrochloride for Injection.
Cardiac Function
Cardiotoxicity is a known risk of anthracycline treatment. Anthracycline-induced cardiac toxicity may be manifested by early (or acute) or late (delayed) events. Early cardiac toxicity of epirubicin consists mainly of sinus tachycardia and/or ECG abnormalities such as non-specific ST-T wave changes, but tachyarrhythmias, including premature ventricular contractions and ventricular tachycardia, bradycardia, as well as atrioventricular and bundle-branch block have also been reported. These effects do not usually predict subsequent development of delayed cardiotoxicity, are rarely of clinical importance, and are generally not considered an indication for the suspension of epirubicin treatment. Delayed cardiac toxicity results from a characteristic cardiomyopathy that is manifested by reduced LVEF and/or signs and symptoms of congestive heart failure (CHF) such as tachycardia, dyspnea, pulmonary edema, dependent edema, hepatomegaly, ascites, pleural effusion, gallop rhythm. Life-threatening CHF is the most severe form of anthracycline-induced cardiomyopathy. This toxicity appears to be dependent on the cumulative dose of Epirubicin Hydrochloride for Injection and represents the cumulative dose-limiting toxicity of the drug. If it occurs, delayed cardiotoxicity usually develops late in the course of therapy with Epirubicin Hydrochloride for Injection or within 2 to 3 months after completion of treatment, but later events (several months to years after treatment termination) have been reported.
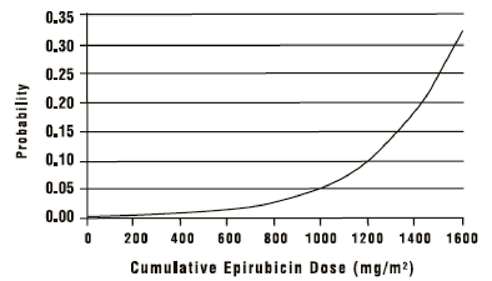
In a retrospective survey, including 9144 patients, mostly with solid tumors in advanced stages, the probability of developing CHF increased with increasing cumulative doses of Epirubicin Hydrochloride for Injection (Figure 5). The estimated risk of epirubicin-treated patients developing clinically evident CHF was 0.9% at a cumulative dose of 550 mg/m2, 1.6% at 700 mg/m2, and 3.3% at 900 mg/m2. The risk of developing CHF in the absence of other cardiac risk factors increased steeply after an epirubicin cumulative dose of 900 mg/m2.
Figure 5. Risk of CHF in 9144 Patients Treated with Epirubicin
In another retrospective survey of 469 epirubicin-treated patients with metastatic or early breast cancer, the reported risk of CHF was comparable to that observed in the larger study of over 9000 patients.
Given the risk of cardiomyopathy, a cumulative dose of 900 mg/m2 Epirubicin Hydrochloride for Injection should be exceeded only with extreme caution. Risk factors (active or dormant cardiovascular disease, prior or concomitant radiotherapy to the mediastinal/pericardial area, previous therapy with other anthracyclines or anthracenediones, concomitant use of other drugs with the ability to suppress cardiac contractility) may increase the risk of cardiac toxicity. Although not formally tested, it is probable that the toxicity of epirubicin and other anthracyclines or anthracenediones is additive. Cardiac toxicity with Epirubicin Hydrochloride for Injection may occur at lower cumulative doses whether or not cardiac risk factors are present.
Although endomyocardial biopsy is recognized as the most sensitive diagnostic tool to detect anthracycline-induced cardiomyopathy, this invasive examination is not practically performed on a routine basis. Electrocardiogram (ECG) changes such as dysrhythmias, a reduction of the QRS voltage, or a prolongation beyond normal limits of the systolic time interval may be indicative of anthracycline-induced cardiomyopathy, but ECG is not a sensitive or specific method for following anthracycline-related cardiotoxicity. The risk of serious cardiac impairment may be decreased through regular monitoring of LVEF during the course of treatment with prompt discontinuation of Epirubicin Hydrochloride for Injection at the first sign of impaired function. The preferred method for repeated assessment of cardiac function is evaluation of LVEF measured by multi-gated radionuclide angiography (MUGA) or echocardiography (ECHO). A baseline cardiac evaluation with an ECG and a MUGA scan or an ECHO is recommended, especially in patients with risk factors for increased cardiac toxicity. Repeated MUGA or ECHO determinations of LVEF should be performed, particularly with higher, cumulative anthracycline doses. The technique used for assessment should be consistent through follow-up. In patients with risk factors, particularly prior anthracycline or anthracenedione use, the monitoring of cardiac function must be particularly strict and the risk-benefit of continuing treatment with Epirubicin Hydrochloride for Injection in patients with impaired cardiac function must be carefully evaluated.
Secondary Leukemia
The occurrence of secondary acute myelogenous leukemia, with or without a preleukemic phase, has been reported in patients treated with anthracyclines. Secondary leukemia is more common when such drugs are given in combination with DNA-damaging antineoplastic agents, when patients have been heavily pretreated with cytotoxic drugs, or when doses of the anthracyclines have been escalated. These leukemias can have a short 1- to 3- year latency period. An analysis of 7110 patients who received adjuvant treatment with epirubicin in controlled clinical trials as a component of poly-chemotherapy regimens for early breast cancer, showed a cumulative risk of secondary acute myelogenous leukemia or myelodysplastic syndrome (AML/MDS) of about 0.27% (approximate 95% CI, 0.14 to 0.40) at 3 years, 0.46% (approximate 95% CI, 0.28 to 0.65) at 5 years and 0.55% (approximate 95% CI, 0.33 to 0.78) at 8 years. The risk of developing AML/MDS increased with increasing epirubicin cumulative doses as shown in Figure 6.
Figure 6. Risk of AML/MDS in 7110 Patients Treated with Epirubicin
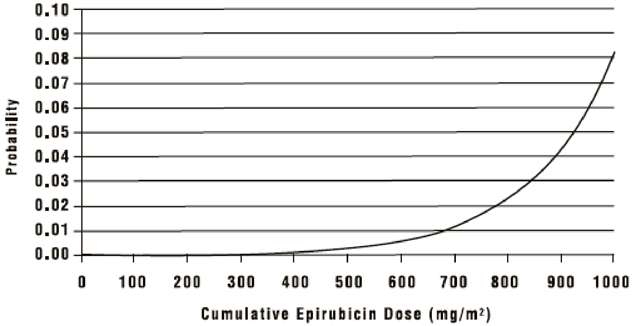
The cumulative probability of developing AML/MDS was found to be particularly increased in patients who received more than the maximum recommended cumulative dose of epirubicin (720 mg/m2) or cyclophosphamide (6,300 mg/m2), as shown in Table 4.
|
Years from |
Cumulative Probability of Developing AML/MDS |
|||
|
Cyclophosphamide Cumulative Dose |
Cyclophosphamide Cumulative Dose |
|||
|
Epirubicin |
Epirubicin Cumulative |
Epirubicin |
Epirubicin Cumulative |
|
|
3 |
0.12 |
0 |
0.12 |
4.37 |
|
5 |
0.15 |
2.38 |
0.31 |
4.97 |
|
8 |
0.37 |
2.38 |
0.31 |
4.97 |
Epirubicin Hydrochloride for Injection is mutagenic, clastogenic, and carcinogenic in animals (see next section, Carcinogenesis, Mutagenesis and Impairment of Fertility ).
Carcinogenesis, Mutagenesis and Impairment of Fertility
Treatment-related acute myelogenous leukemia has been reported in women treated with epirubicin-based adjuvant chemotherapy regimens (see above section, WARNINGS, Secondary Leukemia ). Conventional long-term animal studies to evaluate the carcinogenic potential of epirubicin have not been conducted, but intravenous administration of a single 3.6 mg/kg epirubicin dose to female rats (about 0.2 times the maximum recommended human dose on a body surface area basis) approximately doubled the incidence of mammary tumors (primarily fibroadenomas) observed at 1 year. Administration of 0.5 mg/kg epirubicin intravenously to rats (about 0.025 times the maximum recommended human dose on a body surface area basis) every 3 weeks for ten doses increased the incidence of subcutaneous fibromas in males over an 18-month observation period. In addition, subcutaneous administration of 0.75 or 1.0 mg/kg/day (about 0.015 times the maximum recommended human dose on a body surface area basis) to newborn rats for 4 days on both the first and tenth day after birth for a total of eight doses increased the incidence of animals with tumors compared to controls during a 24-month observation period.
Epirubicin was mutagenic in vitro to bacteria (Ames test) either in the presence or absence of metabolic activation and to mammalian cells (HGPRT assay in V79 Chinese hamster lung fibroblasts) in the absence but not in the presence of metabolic activation. Epirubicin was clastogenic in vitro (chromosome aberrations in human lymphocytes) both in the presence and absence of metabolic activation and was also clastogenic in vivo (chromosome aberration in mouse bone marrow).
In fertility studies in rats, males were given epirubicin daily for 9 weeks and mated with females that were given epirubicin daily for 2 weeks prior to mating and through Day 7 of gestation. When 0.3 mg/kg/day (about 0.015 times the maximum recommended human single dose on a body surface area basis) was administered to both sexes, no pregnancies resulted. No effects on mating behavior or fertility were observed at 0.1 mg/kg/day, but male rats had atrophy of the testes and epididymis, and reduced spermatogenesis. The 0.1 mg/kg/day dose also caused embryolethality. An increased incidence of fetal growth retardation was observed in these studies at 0.03 mg/kg/day (about 0.0015 times the maximum recommended human single dose on a body surface area basis). Multiple daily doses of epirubicin to rabbits and dogs also caused atrophy of male reproductive organs. Single 20.5 and 12 mg/kg doses of intravenous epirubicin caused testicular atrophy in mice and rats, respectively (both approximately 0.5 times the maximum recommended human dose on a body surface area basis). A single dose of 16.7 mg/kg epirubicin caused uterine atrophy in rats.
Although experimental data are not available, Epirubicin Hydrochloride for Injection could induce chromosomal damage in human spermatozoa due to its genotoxic potential. Men undergoing treatment with Epirubicin Hydrochloride for Injection should use effective contraceptive methods. Epirubicin Hydrochloride for Injection may cause irreversible amenorrhea (premature menopause) in premenopausal women.
Liver Function
The major route of elimination of epirubicin is the hepatobiliary system (see CLINICAL PHARMACOLOGY, Pharmacokinetics in Special Populations ). Serum total bilirubin and AST levels should be evaluated before and during treatment with Epirubicin Hydrochloride for Injection. Patients with elevated bilirubin or AST may experience slower clearance of drug with an increase in overall toxicity. Lower doses are recommended in these patients (see DOSAGE AND ADMINISTRATION ). Patients with severe hepatic impairment have not been evaluated; therefore, epirubicin should not be used in this patient population.
Renal Function
Serum creatinine should be assessed before and during therapy. Dosage adjustment is necessary in patients with serum creatinine >5 mg/dL (see DOSAGE AND ADMINISTRATION ). Patients undergoing dialysis have not been studied.
Tumor-Lysis Syndrome
As with other cytotoxic agents, Epirubicin Hydrochloride for Injection may induce hyperuricemia as a consequence of the extensive purine catabolism that accompanies drug-induced rapid lysis of highly chemosensitive neoplastic cells (tumor lysis syndrome). Other metabolic abnormalities may also occur. While not generally a problem in patients with breast cancer, physicians should consider the potential for tumor-lysis syndrome in potentially susceptible patients and should consider monitoring serum uric acid, potassium, calcium, phosphate, and creatinine immediately after initial chemotherapy administration. Hydration, urine alkalinization, and prophylaxis with allopurinol to prevent hyperuricemia may minimize potential complications of tumor-lysis syndrome.
Pregnancy - Category D
Epirubicin Hydrochloride for Injection may cause fetal harm when administered to a pregnant woman. Administration of 0.8 mg/kg/day intravenously of epirubicin to rats (about 0.04 times the maximum recommended single human dose on a body surface area basis) during Days 5 to 15 of gestation was embryotoxic (increased resorptions and post-implantation loss) and caused fetal growth retardation (decreased body weight), but was not teratogenic up to this dose. Administration of 2 mg/kg/day intravenously of epirubicin to rats (about 0.1 times the maximum recommended single human dose on a body surface area basis) on Days 9 and 10 of gestation was embryotoxic (increased late resorptions, post-implantation losses, and dead fetuses; and decreased live fetuses), retarded fetal growth (decreased body weight), and caused decreased placental weight. This dose was also teratogenic, causing numerous external (anal atresia, misshapen tail, abnormal genital tubercle), visceral (primarily gastrointestinal, urinary, and cardiovascular systems), and skeletal (deformed long bones and girdles, rib abnormalities, irregular spinal ossification) malformations. Administration of intravenous epirubicin to rabbits at doses up to 0.2 mg/kg/day (about 0.02 times the maximum recommended single human dose on a body surface area basis) during Days 6 to 18 of gestation was not embryotoxic or teratogenic, but a maternally toxic dose of 0.32 mg/kg/day increased abortions and delayed ossification. Administration of a maternally toxic intravenous dose of 1 mg/kg/day epirubicin to rabbits (about 0.1 times the maximum recommended single human dose on a body surface area basis) on Days 10 to 12 of gestation induced abortion, but no other signs of embryofetal toxicity or teratogenicity were observed. When doses up to 0.5 mg/kg/day epirubicin were administered to rat dams from Day 17 of gestation to Day 21 after delivery (about 0.025 times the maximum recommended single human dose on a body surface area basis), no permanent changes were observed in the development, functional activity, behavior, or reproductive performance of the offspring.
There are no adequate and well-controlled studies in pregnant women. Two pregnancies have been reported in women taking epirubicin. A 34-year-old woman, 28 weeks pregnant at her diagnosis of breast cancer, was treated with cyclophosphamide and epirubicin every 3 weeks for 3 cycles. She received the last dose at 34 weeks of pregnancy and delivered a healthy baby at 35 weeks. A second 34-year-old woman with breast cancer metastatic to the liver was randomized to FEC-50 but was removed from study because of pregnancy. She experienced a spontaneous abortion. If epirubicin is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
PRECAUTIONS
General
Epirubicin Hydrochloride for Injection is administered by intravenous infusion. Venous sclerosis may result from an injection into a small vessel or from repeated injections into the same vein. Extravasation of epirubicin during the infusion may cause local pain, severe tissue lesions (vesication, severe cellulitis) and necrosis. It is recommended that Epirubicin Hydrochloride for Injection be slowly administered into the tubing of a freely running intravenous infusion. Patients receiving initial therapy at the recommended starting doses of 100 to 120 mg/m2 should generally have epirubicin infused over 15 to 20 minutes. For patients who require lower epirubicin starting doses due to organ dysfunction or who require modification of epirubicin doses during therapy, the epirubicin infusion time may be proportionally decreased, but should not be less than 3 minutes (see DOSAGE AND ADMINISTRATION, Preparation of Infusion Solution ). If possible, veins over joints or in extremities with compromised venous or lymphatic drainage should be avoided. A burning or stinging sensation may be indicative of perivenous infiltration, and the infusion should be immediately terminated and restarted in another vein. Perivenous infiltration may occur without causing pain.
Facial flushing, as well as local erythematous streaking along the vein, may be indicative of excessively rapid administration. It may precede local phlebitis or thrombophlebitis.
Patients administered the 120 mg/m2 regimen of Epirubicin Hydrochloride for Injection as a component of combination chemotherapy should also receive prophylactic antibiotic therapy with trimethoprim-sulfamethoxazole (e.g., Septra®, Bactrim®)* or a fluoroquinolone (see CLINICAL STUDIES, Early Breast Cancer , and DOSAGE AND ADMINISTRATION ).
Epirubicin is emetigenic. Antiemetics may reduce nausea and vomiting; prophylactic use of antiemetics should be considered before administration of Epirubicin Hydrochloride for Injection, particularly when given in conjunction with other emetigenic drugs.
As with other anthracyclines, administration of Epirubicin Hydrochloride for Injection after previous radiation therapy may induce an inflammatory recall reaction at the site of the irradiation.
As with other cytotoxic agents, thrombophlebitis and thromboembolic phenomena, including pulmonary embolism (in some cases fatal) have been coincidentally reported with the use of epirubicin.
Information for Patients
Patients should be informed of the expected adverse effects of epirubicin, including gastrointestinal symptoms (nausea, vomiting, diarrhea, and stomatitis) and potential neutropenic complications. Patients should consult their physician if vomiting, dehydration, fever, evidence of infection, symptoms of CHF, or injection-site pain occurs following therapy with Epirubicin Hydrochloride for Injection. Patients should be informed that they will almost certainly develop alopecia. Patients should be advised that their urine may appear red for 1 to 2 days after administration of Epirubicin Hydrochloride for Injection and that they should not be alarmed. Patients should understand that there is a risk of irreversible myocardial damage associated with treatment with Epirubicin Hydrochloride for Injection, as well as a risk of treatment-related leukemia. Because epirubicin may induce chromosomal damage in sperm, men undergoing treatment with Epirubicin Hydrochloride for Injection should use effective contraceptive methods. Women treated with Epirubicin Hydrochloride for Injection may develop irreversible amenorrhea, or premature menopause.
Laboratory Testing
See WARNINGS . Blood counts, including absolute neutrophil counts, and liver function should be assessed before and during each cycle of therapy with epirubicin. Repeated evaluations of LVEF should be performed during therapy.
Drug Interactions
Epirubicin Hydrochloride for Injection when used in combination with other cytotoxic drugs may show on-treatment additive toxicity, especially hematologic and gastrointestinal effects.
Concomitant use of Epirubicin Hydrochloride for Injection with other cardioactive compounds that could cause heart failure (e.g., calcium channel blockers), requires close monitoring of cardiac function throughout treatment.
There are few data regarding the coadministration of radiation therapy and epirubicin. In adjuvant trials of epirubicin-containing CEF-120 or FEC-100 chemotherapies, breast irradiation was delayed until after chemotherapy was completed. This practice resulted in no apparent increase in local breast cancer recurrence relative to published accounts in the literature. A small number of patients received epirubicin-based chemotherapy concomitantly with radiation therapy but had chemotherapy interrupted in order to avoid potential overlapping toxicities. It is likely that use of epirubicin with radiotherapy may sensitize tissues to the cytotoxic actions of irradiation. Administration of Epirubicin Hydrochloride for Injection after previous radiation therapy may induce an inflammatory recall reaction at the site of the irradiation.
Epirubicin is extensively metabolized by the liver. Changes in hepatic function induced by concomitant therapies may affect epirubicin metabolism, pharmacokinetics, therapeutic efficacy, and/or toxicity.
Cimetidine increased the AUC of epirubicin by 50%. Cimetidine treatment should be stopped during treatment with Epirubicin Hydrochloride for Injection (see CLINICAL PHARMACOLOGY ).
Drug-Laboratory Test Interactions
There are no known interactions between Epirubicin Hydrochloride for Injection and laboratory tests.
Carcinogenesis, Mutagenesis & Impairment of Fertility
See WARNINGS .
Pregnancy
Pregnancy Category D - see WARNINGS .
Nursing Mothers
Epirubicin was excreted into the milk of rats treated with 0.50 mg/kg/day of epirubicin during peri- and postnatal periods. It is not known whether epirubicin is excreted in human milk. Because many drugs, including other anthracyclines, are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from epirubicin, mothers should discontinue nursing prior to taking this drug.
Geriatric Use
Although a lower starting dose of Epirubicin Hydrochloride for Injection was not used in trials in elderly female patients, particular care should be taken in monitoring toxicity when Epirubicin Hydrochloride for Injection is administered to female patients ≥ 70 years of age. (See CLINICAL PHARMACOLOGY, Pharmacokinetics in Special Populations. )
Pediatric Use
The safety and effectiveness of epirubicin in pediatric patients have not been established in adequate and well-controlled clinical trials. Pediatric patients may be at greater risk for anthracycline-induced acute manifestations of cardiotoxicity and for chronic CHF.
EPIRUBICIN HYDROCHLORIDE ADVERSE REACTIONS
On-Study Events
Integrated safety data are available from two studies (Studies MA-5 and GFEA-05, see CLINICAL STUDIES ) evaluating epirubicin-containing combination regimens in patients with early breast cancer. Of the 1260 patients treated in these studies, 620 patients received the higher-dose epirubicin regimen (FEC-100/CEF-120), 280 patients received the lower-dose epirubicin regimen (FEC-50), and 360 patients received CMF. Serotonin-specific antiemetic therapy and colony-stimulating factors were not used in these trials. Clinically relevant acute adverse events are summarized in Table 5.
| Event | % of Patients | |||||
|
FEC-100/CEF-120 (N=260) |
FEC-50 (N=280) |
CMF (N=360) |
||||
| Grades 1-4 | Grades 3/4 | Grades 1-4 | Grades 3/4 | Grades 1-4 | Grades 3/4 | |
|
Hematologic Leukopenia Neutropenia Anemia Thrombocytopenia |
80.3 80.3 72.2 48.8 |
58.6 67.2 5.8 5.4 |
49.6 53.9 12.9 4.6 |
1.5 10.5 0 0 |
98.1 95.8 70.9 51.4 |
60.3 78.1 0.9 3.6 |
|
Endocrine Amenorrhea Hot flashes |
71.8 38.9 |
0 4.0 |
69.3 5.4 |
0 0 |
67.7 69.1 |
0 6.4 |
|
Body as a Whole Lethargy Fever |
45.8 5.2 |
1.9 0 |
1.1 1.4 |
0 0 |
72.7 4.5 |
0.3 0 |
|
Gastrointestinal Nausea/vomiting Mucositis Diarrhea Anorexia |
92.4 58.5 24.8 2.9 |
25.0 8.9 0.8 0 |
83.2 9.3 7.1 1.8 |
22.1 0 0 0 |
85.0 52.9 50.7 5.8 |
6.4 1.9 2.8 0.3 |
|
Infection Infection Febrile nuetropenia |
21.5 N/A |
1.6 6.1 |
15.0 0 |
25.9 N/A |
25.9 N/A |
0.6 1.1 |
|
Ocular Conjunctivitis/keratitis |
14.8 |
0 |
1.1 |
0 |
38.4 |
0 |
|
Skin Alopecia Local toxicity Rash/itch Skin changes |
95.5 19.5 8.9 4.7 |
56.6 0.3 0.3 0 |
69.6 2.5 1.4 0.7 |
19.3 0.4 0 0 |
84.4 8.1 14.2 7.2 |
6.7 0 0 0 |
|
FEC & CEF = cyclophosphamide + epirubicin + fluorouracil; CMF = cyclophosphamide + methotrexate + fluorouracil, N/A = not available Grade 1 or 2 changes in transaminase levels were observed but were more frequently seen with CMF than with CEF. |
||||||
Delayed Events
Table 6 describes the incidence of delayed adverse events in patients participating in the MA-5 and GFEA-05 trials.
| % of Patients | |||
|---|---|---|---|
| Event |
FEC/100/CEF-120
(N=620) |
FEC-50
(N=280) |
CMF
(N=360 |
|
Cardiac events Asymptomatic drops in LVEF CHF |
2.1* 1.5 |
1.4 0.4 |
0.8* 0.3 |
|
Leukemia AML |
0.8 |
0 |
0.3 |
|
*In study MA-5 cardiac function was not monitored after 5 years. |
|||
Two cases of acute lymphoid leukemia (ALL) were also observed in patients receiving epirubicin. However, an association between anthracyclines such as epirubicin and ALL has not been clearly established.
Overview of Acute and Delayed Toxicities
Hematologic
See WARNINGS .
Gastrointestinal
A dose-dependent mucositis (mainly oral stomatitis, less often esophagitis) may occur in patients treated with epirubicin. Clinical manifestations of mucositis may include a pain or burning sensation, erythema, erosions, ulcerations, bleeding, or infections. Mucositis generally appears early after drug administration and, if severe, may progress over a few days to mucosal ulcerations; most patients recover from this adverse event by the third week of therapy. Hyperpigmentation of the oral mucosa may also occur.
Nausea, vomiting, and occasionally diarrhea and abdominal pain can also occur. Severe vomiting and diarrhea may produce dehydration. Antiemetics may reduce nausea and vomiting; prophylactic use of antiemetics should be considered before therapy (see PRECAUTIONS ).
Cutaneous and Hypersensitivity Reactions
Alopecia occurs frequently, but is usually reversible, with hair regrowth occurring within 2 to 3 months from the termination of therapy. Flushes, skin and nail hyperpigmentation, photosensitivity, and hypersensitivity to irradiated skin (radiation-recall reaction) have been observed. Urticaria and anaphylaxis have been reported in patients treated with epirubicin; signs and symptoms of these reactions may vary from skin rash and pruritus to fever, chills, and shock.
Cardiovascular
See WARNINGS .
Secondary Leukemia
See WARNINGS .
Injection-Site Reactions
See PRECAUTIONS .
OVERDOSAGE
A 36-year-old man with non-Hodgkin’s lymphoma received a daily 95 mg/m2 dose of Epirubicin Hydrochloride for Injection for 5 consecutive days. Five days later, he developed bone marrow aplasia, grade 4 mucositis, and gastrointestinal bleeding. No signs of acute cardiac toxicity were observed. He was treated with antibiotics, colony-stimulating factors, and antifungal agents, and recovered completely. A 63-year-old woman with breast cancer and liver metastasis received a single 320 mg/m2 dose of Epirubicin Hydrochloride for Injection. She was hospitalized with hyperthermia and developed multiple organ failure (respiratory and renal), with lactic acidosis, increased lactate dehydrogenase, and anuria. Death occurred within 24 hours after administration of Epirubicin Hydrochloride for Injection. Additional instances of administration of doses higher than recommended have been reported at doses ranging from 150 to 250 mg/m2. The observed adverse events in these patients were qualitatively similar to known toxicities of epirubicin. Most of the patients recovered with appropriate supportive care.
If an overdose occurs, supportive treatment (including antibiotic therapy, blood and platelet transfusions, colony-stimulating factors, and intensive care as needed) should be provided until the recovery of toxicities. Delayed CHF has been observed months after anthracycline administration. Patients must be observed carefully over time for signs of CHF and provided with appropriate supportive therapy.
EPIRUBICIN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
Epirubicin Hydrochloride for Injection is administered to patients by intravenous infusion. Epirubicin Hydrochloride for Injection is given in repeated 3- to 4-week cycles. The total dose of Epirubicin Hydrochloride for Injection may be given on Day 1 of each cycle or divided equally and given on Days 1 and 8 of each cycle. The recommended dosages of Epirubicin Hydrochloride for Injection are as follows:
Starting Doses
The recommended starting dose of Epirubicin Hydrochloride for Injection is 100 to 120 mg/m2. The following regimens were used in the trials supporting use of Epirubicin Hydrochloride for Injection as a component of adjuvant therapy in patients with axillary-node positive breast cancer:
CEF-120: Cyclophosphamide 75 mg/m2 PO D 1-14
Epirubicin Hydrochloride for Injection 60 mg/m2 IV D 1, 8
5-Fluorouracil 500 mg/m2 IV D 1, 8
Repeated every 28 days for 6 cycles
FEC-100: 5-Fluorouracil 500 mg/m2
Epirubicin Hydrochloride for Injection 100 mg/m2
Cyclophosphamide 500 mg/m2
All drugs administered intravenously on Day 1 and repeated every 21 days for 6 cycles
Patients administered the 120 mg/m2 regimen of Epirubicin Hydrochloride for Injection also received prophylactic antibiotic therapy with trimethoprim-sulfamethoxazole (e.g., Septra®, Bactrim®)* or a fluoroquinolone.
Bone Marrow Dysfunction
Consideration should be given to administration of lower starting doses (75 to 90 mg/m2) for heavily pretreated patients, patients with pre-existing bone marrow depression, or in the presence of neoplastic bone marrow infiltration (see WARNINGS and PRECAUTIONS ).
Hepatic Dysfunction
Definitive recommendations regarding use of Epirubicin Hydrochloride for Injection in patients with hepatic dysfunction are not available because patients with hepatic abnormalities were excluded from participation in adjuvant trials of FEC-100/CEF-120 therapy. In patients with elevated serum AST or serum total bilirubin concentrations, the following dose reductions were recommended in clinical trials, although few patients experienced hepatic impairment:
-
Bilirubin 1.2 to 3 mg/dL or AST 2 to 4 times upper limit of normal
1/2 of recommended starting dose
-
Bilirubin > 3 mg/dL or AST > 4 times upper limit of normal
1/4 of recommended starting dose
Information regarding experience in patients with hepatic dysfunction is provided in CLINICAL PHARMACOLOGY, Pharmacokinetics in Special Populations .
Renal Dysfunction
While no specific dose recommendation can be made based on the limited available data in patients with renal impairment, lower doses should be considered in patients with severe renal impairment (serum creatinine > 5 mg/dL).
Dose Modifications
Dosage adjustments after the first treatment cycle should be made based on hematologic and nonhematologic toxicities. Patients experiencing during treatment cycle nadir platelet counts <50,000/mm3, absolute neutrophil counts (ANC) <250/mm3, neutropenic fever, or Grades 3/4 nonhematologic toxicity should have the Day 1 dose in subsequent cycles reduced to 75% of the Day 1 dose given in the current cycle. Day 1 chemotherapy in subsequent courses of treatment should be delayed until platelet counts are ≥ 100,000/mm3, ANC ≥ 1500/mm3, and nonhematologic toxicities have recovered to ≤ Grade 1.
For patients receiving a divided dose of Epirubicin Hydrochloride for Injection (Day 1 and Day 8), the Day 8 dose should be 75% of Day 1 if platelet counts are 75,000 to 100,000/mm3 and ANC is 1000 to 1499/mm3. If Day 8 platelet counts are <75,000/mm3, ANC <1000/mm3, or Grade 3/4 nonhematologic toxicity has occurred, the Day 8 dose should be omitted.
Preparation & Administration Precautions
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Procedures normally used for proper handling and disposal of anticancer drugs should be considered for use with Epirubicin Hydrochloride for Injection. Several guidelines on this subject have been published.1-8
Protective Measures
The following protective measures should be taken when handling Epirubicin Hydrochloride for Injection:
-
Personnel should be trained in appropriate techniques for reconstitution and handling.
-
Pregnant staff should be excluded from working with this drug.
-
Personnel handling Epirubicin Hydrochloride for Injection should wear protective clothing: goggles, gowns and disposable gloves and masks.
-
A designated area should be defined for syringe preparation (preferably under a laminar flow system), with the work surface protected by disposable, plastic-backed, absorbent paper.
-
All items used for reconstitution, administration or cleaning (including gloves) should be placed in high-risk, waste-disposal bags for high temperature incineration.
Spillage or leakage should be treated with dilute sodium hypochlorite (1% available chlorine) solution, preferably by soaking, and then water. All contaminated and cleaning materials should be placed in high-risk, waste-disposal bags for incineration. Accidental contact with the skin or eyes should be treated immediately by copious lavage with water, or soap and water, or sodium bicarbonate solution. However, do not abrade the skin by using a scrub brush. Medical attention should be sought. Always wash hands after removing gloves.
Incompatibilities
Prolonged contact with any solution of an alkaline pH should be avoided as it will result in hydrolysis of the drug. Epirubicin Hydrochloride for Injection should not be mixed with heparin or fluorouracil due to chemical incompatibility that may lead to precipitation.
Epirubicin Hydrochloride for Injection can be used in combination with other antitumor agents, but it is not recommended that it be mixed with other drugs in the same syringe.
Preparation of Infusion Solution
Reconstitution
Prior to use, Epirubicin Hydrochloride for Injection 50 mg and 200 mg vials must be reconstituted with 25 mL and 100 mL, respectively, of Sterile Water for Injection, USP, resulting in a solution concentration of 2 mg/mL with a pH of 4.7 to 5.0. Shake vigorously. It may take up to 4 minutes for epirubicin hydrochloride to completely dissolve. Reconstituted solutions are stable for 24 hours when stored at 2 to 8°C (36 to 46°F) and protected from light, or 25°C (77°F) in normal lighting conditions.
Epirubicin Hydrochloride for Injection can be further diluted with Sterile Water for Injection, USP.
Administration
Epirubicin Hydrochloride for Injection should be administered into the tubing of a freely flowing intravenous infusion (0.9% sodium chloride or 5% glucose solution). Patients receiving initial therapy at the recommended starting doses of 100 to 120 mg/m2 should generally have epirubicin infused over 15 to 20 minutes. For patients who require lower epirubicin starting doses due to organ dysfunction or who require modification of epirubicin doses during therapy, the epirubicin infusion time may be proportionally decreased, but should not be less than 3 minutes. This technique is intended to minimize the risk of thrombosis or perivenous extravasation, which could lead to severe cellulitis, vesication, or tissue necrosis. A direct push injection is not recommended due to the risk of extravasation, which may occur even in the presence of adequate blood return upon needle aspiration. Venous sclerosis may result from injection into small vessels or repeated injections into the same vein (see PRECAUTIONS ). Epirubicin Hydrochloride for Injection should be used within 24 hours of first penetration of the rubber stopper. Discard any unused solution.
HOW SUPPLIED
Epirubicin Hydrochloride for Injection is available in single-use vials containing 50 mg and 200 mg epirubicin hydrochloride.
50 mg/vial NDC 61703-347-35
200 mg/vial NDC 61703-348-59
Store unopened vials at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Protect from light. Discard unused portion. Store Upright.
*Septra® and Bactrim® are registered trademarks of Monarch Pharmaceuticals Inc. and Mutual Pharmaceutical Company, Inc., respectively.
REFERENCES
-
ONS Clinical Practice Committee. Cancer Chemotherapy Guidelines and Recommendations for Practice. Pittsburgh, PA: Oncology Nursing Society; 1999: 32-41.
-
Recommendations for the Safe Handling of Parenteral Antineoplastic Drugs. Washington, DC: Division of Safety, Clinical Center Pharmacy Department and Cancer Nursing Services, National Institutes of Health; 1992 US Dept of Health and Human Services. Public Health Service Publication NIH 92-2621.
-
AMA Council on Scientific Affairs. Guidelines for Handling Parenteral Antineoplastics. JAMA 1985; 253(11):1590-1592.
-
National Study Commission on Cytotoxic Exposure - Recommendations for Handling of Cytotoxic Agents. 1987. Available from Louis P. Jeffrey, ScD., Chairman, National Study Commission on Cytotoxic Exposure, Massachusetts College of Pharmacy and Allied Health Sciences, 179 Longwood Avenue, Boston, MA 02115.
-
Clinical Oncology Society of Australia, Guidelines and Recommendations for Safe Handling of Antineoplastic Agents. Med J Australia 1983; 1:426-428.
-
Jones RB, Frank R, Mass T. Safe Handling of Chemotherapeutic Agents: A Report from the Mount Sinai Medical Center. CA-A Cancer J for Clin 1983; 33:258-263.
-
American Society of Hospital Pharmacists. ASHP Technical Assistance Bulletin on Handling Cytotoxic and Hazardous Drugs. Am J Hosp Pharm 1990; 47:1033-1049.
-
Controlling Occupational Exposure to Hazardous Drugs (OSHA Work-Practice Guidelines). Am J Health-Syst Pharm 1996; 53: 1669-1685.
Hospira, Inc.
Lake Forest, IL 60045
Product of Australia
Revision November 2007
483480
483478
481517

Epirubicin HydrochlorideEPIRUBICIN HYDROCHLORIDE INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Epirubicin HydrochlorideEPIRUBICIN HYDROCHLORIDE INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||