Epivir
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- EPIVIR DESCRIPTION
- MICROBIOLOGY
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- EPIVIR CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- EPIVIR ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
WARNINGLACTIC ACIDOSIS AND SEVERE HEPATOMEGALY WITH STEATOSIS, INCLUDING FATAL CASES, HAVE BEEN REPORTED WITH THE USE OF NUCLEOSIDE ANALOGUES ALONE OR IN COMBINATION, INCLUDING LAMIVUDINE AND OTHER ANTIRETROVIRALS (SEE WARNINGS).
HUMAN IMMUNODEFICIENCY VIRUS (HIV) COUNSELING AND TESTING SHOULD BE OFFERED TO ALL PATIENTS BEFORE BEGINNING EPIVIR-HBV AND PERIODICALLY DURING TREATMENT (SEE WARNINGS), BECAUSE EPIVIR-HBV TABLETS AND ORAL SOLUTION CONTAIN A LOWER DOSE OF THE SAME ACTIVE INGREDIENT (LAMIVUDINE) AS EPIVIRTABLETS AND ORAL SOLUTION USED TO TREAT HIV INFECTION. IF TREATMENT WITH EPIVIR-HBV IS PRESCRIBED FOR CHRONIC HEPATITIS B FOR A PATIENT WITH UNRECOGNIZED OR UNTREATED HIV INFECTION, RAPID EMERGENCE OF HIV RESISTANCE IS LIKELY BECAUSE OF SUBTHERAPEUTIC DOSE AND INAPPROPRIATE MONOTHERAPY.
SEVERE ACUTE EXACERBATIONS OF HEPATITIS B HAVE BEEN REPORTED IN PATIENTS WHO HAVE DISCONTINUED ANTI-HEPATITIS B THERAPY (INCLUDING EPIVIR-HBV). HEPATIC FUNCTION SHOULD BE MONITORED CLOSELY WITH BOTH CLINICAL AND LABORATORY FOLLOW-UP FOR AT LEAST SEVERAL MONTHS IN PATIENTS WHO DISCONTINUE ANTI-HEPATITIS B THERAPY. IF APPROPRIATE, INITIATION OF ANTI-HEPATITIS B THERAPY MAY BE WARRANTED (SEE WARNINGS).
EPIVIR DESCRIPTION
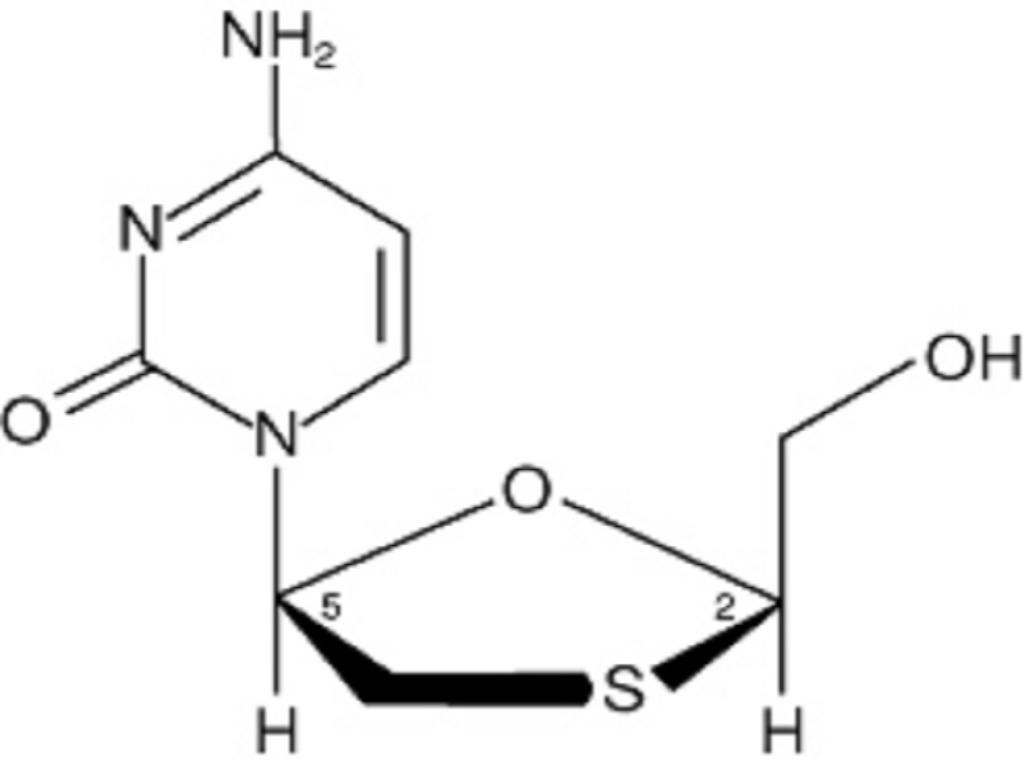
MICROBIOLOGY
Mechanism of Action:
Antiviral Activity:
Resistance:
Cross-Resistance:
CLINICAL PHARMACOLOGY
Pharmacokinetics in Adults:
Special Populations:
Drug Interactions:
INDICATIONS & USAGE
-
● Due to high rates of resistance development in treated patients, initiation of lamivudine treatment should only be considered when the use of an alternative antiviral agent with a higher genetic barrier to resistance is not available or appropriate.
Description of Clinical Studies:
-
● Study 1 was a randomized, double-blind study of EPIVIR-HBV 100 mg once daily versus placebo for 52 weeks followed by a 16-week no-treatment period in treatment-naive US patients.
-
● Study 2 was a randomized, double-blind, 3-arm study that compared EPIVIR-HBV 25 mg once daily versus EPIVIR-HBV 100 mg once daily versus placebo for 52 weeks in Asian patients.
-
● Study 3 was a randomized, partially-blind, 3-arm study conducted primarily in North America and Europe in patients who had ongoing evidence of active chronic hepatitis B despite previous treatment with interferon alfa. The study compared EPIVIR-HBV 100 mg once daily for 52 weeks, followed by either EPIVIR-HBV 100 mg or matching placebo once daily for 16 weeks (Arm 1), versus placebo once daily for 68 weeks (Arm 2). (A third arm using a combination of interferon and lamivudine is not presented here because there was not sufficient information to evaluate this regimen.)
EPIVIR CONTRAINDICATIONS
WARNINGS
Lactic Acidosis/Severe Hepatomegaly With Steatosis:Important Differences Between Lamivudine-Containing Products, HIV Testing, and Risk of Emergence of Resistant HIV:
Posttreatment Exacerbations of Hepatitis:
Pancreatitis:
PRECAUTIONS
General:Emergence of Resistance-Associated HBV Mutations:
Limitations of Populations Studied:
Assessing Patients During Treatment:
Patients With Impaired Renal Function:
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
EPIVIR ADVERSE REACTIONS
Clinical Trials In Chronic Hepatitis B:
Lamivudine in Patients With HIV:
Pediatric Patients With Hepatitis B:
Pediatric Patients With HIV Infection:
Observed During Clinical Practice:
OVERDOSAGE
DOSAGE & ADMINISTRATION
Adults:
Pediatric Patients:
Dose Adjustment:
HOW SUPPLIED
INFORMATION FOR PATIENTS
Advice for the Patient
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

EpivirLamivudine TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!