Ery-Tab
REMEDYREPACK INC.
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ERY-TAB DESCRIPTION
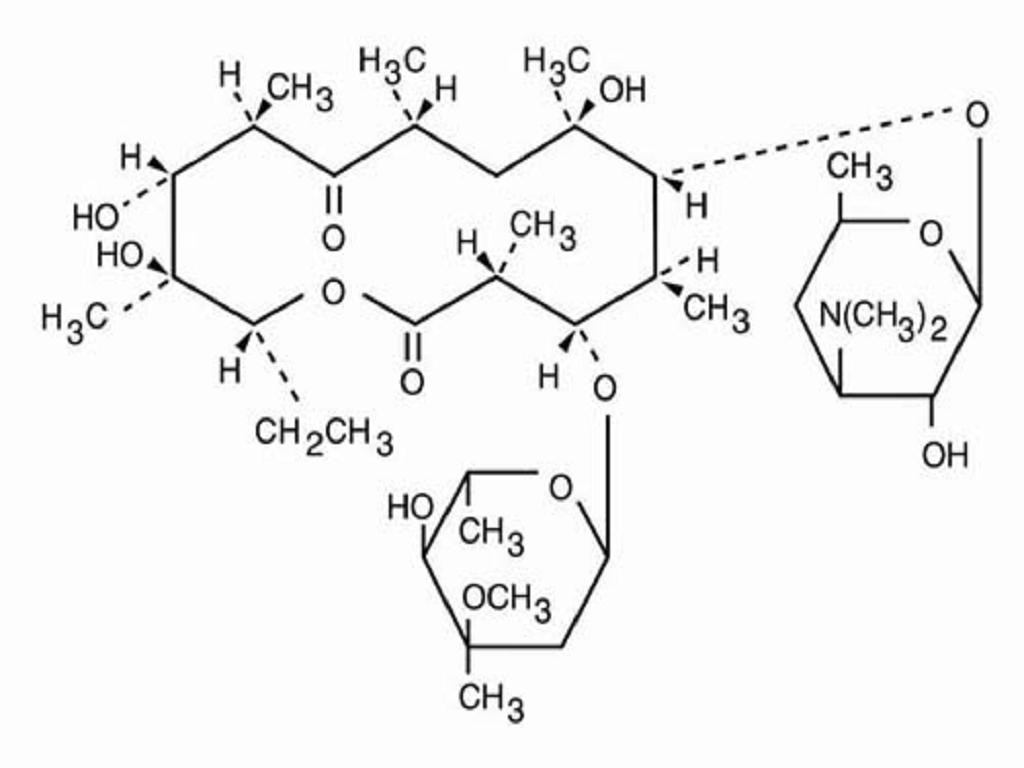
INACTIVE INGREDIENT
CLINICAL PHARMACOLOGY
MICROBIOLOGY
INDICATIONS & USAGE
ERY-TAB CONTRAINDICATIONS
WARNINGS
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including erythromycin, and may range in severity from mild to life threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.PRECAUTIONS
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
ERY-TAB ADVERSE REACTIONS
OVERDOSAGE
DOSAGE & ADMINISTRATION
HOW SUPPLIED
-
● NDC 24338-124-13 bottles of 100
STORAGE AND HANDLING
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Ery-Tab
Erythromycin TABLET
Product Information
|
|
Product Type
|
Human prescription drug label |
Item Code (Source)
|
NDC:49349-773(NDC:24338-124) |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
ERYTHROMYCIN ERYTHROMYCIN |
|
333 mg
|
Product Characteristics
|
|
Color
|
Size
|
Imprint Code
|
Shape
|
|
white |
15 mm |
EH |
OVAL |
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:49349-773-02 |
30 in 1 BLISTER PACK |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
ANDA |
ANDA062298 |
2011-08-19 |
|
|
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!


