ERYTHROMYCIN ETHYLSUCCINATE AND SULFISOXAZOLE ACETYL
ERYTHROMYCIN ETHYLSUCCINATE AND SULFISOXAZOLE ACETYL FOR ORAL SUSPENSION, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- ERYTHROMYCIN ETHYLSUCCINATE AND SULFISOXAZOLE ACETYL DESCRIPTION:
- CLINICAL PHARMACOLOGY:
- ERYTHROMYCIN ETHYLSUCCINATE AND SULFISOXAZOLE ACETYL INDICATIONS AND USAGE:
- ERYTHROMYCIN ETHYLSUCCINATE AND SULFISOXAZOLE ACETYL CONTRAINDICATIONS:
- WARNINGS:
- PRECAUTIONS:
- ERYTHROMYCIN ETHYLSUCCINATE AND SULFISOXAZOLE ACETYL ADVERSE REACTIONS:
- Overdosage:
- ERYTHROMYCIN ETHYLSUCCINATE AND SULFISOXAZOLE ACETYL DOSAGE AND ADMINISTRATION:
- HOW SUPPLIED:
- REFERENCES:
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Revised MARCH 2007
11001190
Rx only
DESCRIPTION:
Erythromycin ethylsuccinate and sulfisoxazole acetyl, when reconstituted with water as directed on the label, the granules form a white, cherry flavored suspension that provides the equivalent of 200 mg erythromycin activity and the equivalent of 600 mg of sulfisoxazole activity per teaspoonful (5 mL).
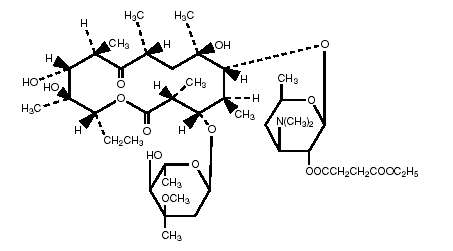
Erythromycin is produced by a strain of Saccaropolyspora erythraea and belongs to the macrolide group of antibiotics. It is basic and readily forms salts and esters. Erythromycin ethylsuccinate is the 2’-ethylsuccinyl ester of erythromycin. It is essentially a tasteless form of the antibiotic suitable for oral administration, particularly in suspension dosage forms. The chemical name is erythromycin 2’-(ethylsuccinate). Erythromycin ethylsuccinate has the following structural formula:
Inactive Ingredients:
Artificial flavor, lactose anhydrous, methylparaben, polysorbate 80, povidone, simethicone, sodium citrate anhydrous and sucrose.
CLINICAL PHARMACOLOGY:
Orally administered erythromycin ethylsuccinate suspensions are readily and reliably absorbed. Erythromycin ethylsuccinate products have demonstrated rapid and consistent absorption in both fasting and nonfasting conditions. However, higher serum concentrations are obtained when these products are given with food. Erythromycin is largely bound to plasma proteins. After absorption, erythromycin diffuses readily into most body fluids. In the absence of meningeal inflammation, low concentrations are normally achieved in the spinal fluid, but the passage of the drug across the blood-brain barrier increases in meningitis. Erythromycin crosses the placental barrier and is excreted in human milk. Erythromycin is not removed by peritoneal dialysis or hemodialysis.
In the presence of normal hepatic function, erythromycin is concentrated in the liver and is excreted in the bile; the effect of hepatic dysfunction on biliary excretion of erythromycin is not known. After oral administration, less than 5% of the administered dose can be recovered in the active form in the urine.
Wide variation in blood levels may result following identical doses of a sulfonamide. Blood levels should be measured in patients receiving these drugs for serious infections. Free sulfonamide blood levels of 50 to 150 mcg/mL may be considered therapeutically effective for most infections, with blood levels of 120 to 150 mcg/mL being optimal for serious infections. The maximum sulfonamide level should be 200 mcg/mL, because adverse reactions occur more frequently above this concentration.
Following oral administration, sulfisoxazole is rapidly and completely absorbed; the small intestine is the major site of absorption, but some of the drug is absorbed from the stomach. Sulfonamides are present in the blood as free, conjugated (acetylated and possibly other forms), and protein-bound forms. The amount present as “free” drug is considered to be the therapeutically active form. Approximately 85% of a dose of sulfisoxazole is bound to plasma proteins, primarily to albumin; 65% to 72% of the unbound portion is in the nonacetylated form.
Maximum plasma concentrations of intact sulfisoxazole following a single 2 g oral dose of sulfisoxazole to healthy adult volunteers ranged from 127 to 211 mcg/mL (mean 169 mcg/mL), and the time of peak plasma concentration ranged from 1 to 4 hours (mean, 2.5 hours). The elimination half-life of sulfisoxazole ranged from 4.6 to 7.8 hours after oral administration. The elimination of sulfisoxazole has been shown to be slower in elderly subjects (63 to 75 years) with diminished renal function (creatine clearance 37 to 68 mL/min).1 After multiple-dose oral administration of 500 mg q.i.d. to healthy volunteers, the average steady-state plasma concentrations of intact sulfisoxazole ranged from 49.9 to 88.8 mcg/mL (mean 63.4 mcg/mL).2
Sulfisoxazole and its acetylated metabolites are excreted primarily by the kidneys through glomerular filtration. Concentrations of sulfisoxazole are considerably higher in the urine than in the blood. The mean urinary recovery following oral administration of sulfisoxazole is 97% within 48 hours; 52% of this is intact drug, and the remainder is the N4-acetylated metabolite.
Sulfisoxazole is distributed only in extracellular body fluids. It is excreted in human milk. It readily crosses the placental barrier. In healthy subjects, cerebrospinal fluid concentrations of sulfisoxazole vary; in patients with meningitis, however, concentrations of free drug in cerebrospinal fluid as high as 94 mcg/mL have been reported.
Microbiology:
This product has been formulated to contain sulfisoxazole for concomitant use with erythromycin.
Erythromycin acts by inhibition of protein synthesis by binding 50 S ribosomal sub-units of susceptible organisms. It does not affect nucleic acid synthesis. Antagonism has been demonstrated in vitro between erythromycin and clindamycin, lincomycin, and chloramphenicol.
The sulfonamides are bacteriostatic agents, and the spectrum of activity is similar for all. Sulfonamides inhibit bacterial synthesis of dihydrofolic acid by preventing the condensation of the pteridine with para-aminobenzoic acid through competitive inhibition of the enzyme dihydropteroate synthetase. Resistant strains have altered dihydropteroate synthetase with reduced affinity for sulfonamides or produce increased quantities of para-aminobenzoic acid.
Susceptibility Testing:
Quantitative methods that require measurement of zone diameter give the most precise estimates of the susceptibility of bacteria to antimicrobial agents. One such standardized single-disc procedure3 has been recommended for use with discs to test susceptibility to erythromycin and sulfisoxazole. Interpretation involves correlation of the zone diameters obtained in the disc test with minimal inhibitory concentration (MIC) values for erythromycin and sulfisoxazole.
If the standardized procedure of disc susceptibility is used, a 15 mcg erythromycin disc should give a zone diameter of at least 18 mm when tested against an erythromycin-susceptible bacterial strain, and a 250-300 mcg sulfisoxazole disc should give a zone diameter of at least 17 mm when tested against a sulfisoxazole-susceptible bacterial strain.
In vitro sulfonamides susceptibility tests are not always reliable because media containing excessive amounts of thymidine are capable of reversing the inhibitory effect of sulfonamides, which may result in false resistant reports. The tests must be carefully coordinated with bacteriological and clinical responses. When the patient is already taking sulfonamides, follow-up cultures should have aminobenzoic acid added to the isolation media but not to subsequent susceptibility test media.
INDICATIONS AND USAGE:
For treatment of ACUTE OTITIS MEDIA in children that is caused by susceptible strains of Haemophilus influenzae.
CONTRAINDICATIONS:
Erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension is contraindicated in the following patient populations:
-
Use in pregnant women at term, in children less than 2 months of age, and in mothers nursing infants less than 2 months of age is contraindicated because sulfonamides may promote kernicterus in the newborn by displacing bilirubin from plasma proteins.
Erythromycin is contraindicated in patients taking terfenadine. (See PRECAUTIONS, Drug Interactions:.)
WARNINGS:
FATALITIES ASSOCIATED WITH THE ADMINISTRATION OF SULFONAMIDES, ALTHOUGH RARE, HAVE OCCURRED DUE TO SEVERE REACTIONS, INCLUDING STEVENS-JOHNSON SYNDROME, TOXIC EPIDERMAL NECROLYSIS, FULMINANT HEPATIC NECROSIS, AGRANULOCYTOSIS, APLASTIC ANEMIA, AND OTHER BLOOD DYSCRASIAS.
SULFONAMIDES, INCLUDING SULFONAMIDE-CONTAINING PRODUCTS SUCH AS ERYTHROMYCIN ETHYLSUCCINATE AND SULFISOXAZOLE ACETYL FOR ORAL SUSPENSION, SHOULD BE DISCONTINUED AT THE FIRST APPEARANCE OF SKIN RASH OR ANY SIGN OF ADVERSE REACTION. In rare instances, a skin rash may be followed by a more severe reaction, such as Stevens-Johnson syndrome, toxic epidermal necrolysis, hepatic necrosis, and serious blood disorders. (See PRECAUTIONS.)
Clinical signs such as sore throat, fever, pallor, rash, purpura, or jaundice may be early indications of serious reactions.
There have been reports of hepatic dysfunction, with or without jaundice, occurring in patients receiving oral erythromycin products.
Cough, shortness of breath, and pulmonary infiltrates are hypersensitivity reactions of the respiratory tract that have been reported in association with sulfonamide treatment.
The sulfonamides should not be used for the treatment of group A beta-hemolytic streptococcal infections. In an established infection, they will not eradicate the streptococcus and, therefore, will not prevent sequelae such as rheumatic fever.
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of “antibiotic-associated colitis”.
After diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against Clostridium difficile colitis.
There have been reports suggesting that erythromycin does not reach the fetus in adequate concentration to prevent congenital syphilis. Infants born to women treated during pregnancy with erythromycin for early syphilis should be treated with an appropriate penicillin regimen.
Rhabdomyolysis with or without renal impairment has been reported in seriously ill patients receiving erythromycin concomitantly with lovastatin. Therefore, patients receiving concomitant lovastatin and erythromycin should be carefully monitored for creatine kinase (CK) and serum transaminase levels. (See package insert for lovastatin.)
PRECAUTIONS:
General:
Erythromycin is principally excreted by the liver. Caution should be exercised when erythromycin is administered to patients with impaired hepatic function. (See CLINICAL PHARMACOLOGY and WARNINGS sections.)
Prolonged or repeated use of erythromycin may result in an overgrowth of nonsusceptible bacteria or fungi. If superinfection occurs, erythromycin should be discontinued and appropriate therapy instituted.
There have been reports that erythromycin may aggravate the weakness of patients with myasthenia gravis.
When indicated, incision and drainage or other surgical procedures should be performed in conjunction with antibiotic therapy.
Sulfonamides should be given with caution to patients with impaired renal or hepatic function and to those with severe allergy or bronchial asthma. In glucose-6-phosphate dehydrogenase-deficient individuals, hemolysis may occur; this reaction is frequently dose-related.
Information For Patients:
Patients should maintain an adequate fluid intake to prevent crystalluria and stone formation.
Laboratory Tests:
Complete blood counts should be done frequently in patients receiving sulfonamides. If a significant reduction in the count of any formed blood element is noted, erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension should be discontinued. Urinalysis with careful microscopic examination and renal function tests should be performed during therapy, particularly for those patients with impaired renal function. Blood levels should be measured in patients receiving a sulfonamide for serious infections. (See INDICATIONS AND USAGE.)
Drug Laboratory Test Interactions:
Erythromycin interferes with the fluorometric determination of urinary catecholamines.
Drug Interactions:
Erythromycin use in patients who are receiving high doses of theophylline may be associated with an increase in serum theophylline levels and potential theophylline toxicity. In case of theophylline toxicity and/or elevated serum theophylline levels, the dose of theophylline should be reduced while the patient is receiving concomitant erythromycin therapy.
Concomitant administration of erythromycin and digoxin has been reported to result in elevated digoxin serum levels.
There have been reports of increased anticoagulant effects when erythromycin and oral anticoagulants were used concomitantly. Increased anticoagulation effects due to this drug may be more pronounced in the elderly.
Concurrent use of erythromycin and ergotamine or dihydroergotamine has been associated in some patients with acute ergot toxicity characterized by severe peripheral vasospasm and dysesthesia.
Erythromycin has been reported to decrease the clearance of triazolam and midazolam and thus may increase the pharmacologic effect of these benzodiazepines.
The use of erythromycin in patients concurrently taking drugs metabolized by the cytochrome P450 system may be associated with elevations in serum levels of these other drugs. There have been reports of interactions of erythromycin with carbamazepine, cyclosporine, hexobarbital, phenytoin, allentanil, diisopyramide, lovastatin, and bromocriptine. Serum concentrations of drugs metabolized by the cytochrome P450 system should be monitored closely in patients concurrently receiving erythromycin.
Erythromycin significantly alters the metabolism of terfenadine when taken concomitantly. Rare cases of serious cardiovascular adverse events, including death, cardiac arrest, torsades de pointes, and other ventricular arrhythmias, have been observed. (See CONTRAINDICATIONS.)
It has been reported that sulfisoxazole may prolong the prothrombin time in patients who are receiving the anticoagulant warfarin. This interaction should be kept in mind when erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension is given to patients already on anticoagulant therapy, and the coagulation time should be reassessed.
It has been proposed that sulfisoxazole competes with thiopental for plasma protein binding. In one study involving 48 patients, intravenous sulfisoxazole resulted in a decrease in the amount of thiopental required for anesthesia and in a shortening of the awakening time. It is not known whether chronic oral doses of sulfisoxazole have a similar effect. Until more is known about this interaction, physicians should be aware that patients receiving sulfisoxazole might require less thiopental for anesthesia.
Sulfonamides can displace methotrexate from plasma protein binding sites, thus increasing free methotrexate concentrations. Studies in man have shown sulfisoxazole infusions to decrease plasma protein-bound methotrexate by one fourth.
Sulfisoxazole can also potentiate the blood-sugar-lowering activity of sulfonylureas.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension has not undergone adequate trials relating to carcinogenicity: each component, however, has been evaluated separately. Long-term (21 month) oral studies conducted in rats with erythromycin ethylsuccinate did not provide evidence of tumorigenicity. Sulfisoxazole was not carcinogenic in either sex when administered to mice by gavage for 103 weeks at dosages up to approximately 18 times the recommended human dose or to rats at 4 times the human dose. Rats appear to be especially susceptible to the goitrogenic effects of sulfonamides, and long-term administration of sulfonamides has resulted in thyroid malignancies in this species.
There are no studies available that adequately evaluate the mutagenic potential of erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension or either of its components. However, sulfisoxazole was not observed to be mutagenic in E. coli Sd-4-73 when tested in the absence of a metabolic activating system. There was no apparent effect on male or female fertility in rats fed erythromycin (base) at levels up to 0.25% of diet.
Erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension has not undergone adequate trials relating to impairment of fertility. In a reproduction study in rats given 7 times the human dose per day of sulfisoxazole, no effects were observed regarding mating behavior, conception rate or fertility index (percent pregnant).
Pregnancy:
Teratogenic Effects:
Pregnancy Category C:
At dosages 7 times the human daily dose, sulfisoxazole was not teratogenic in either rats or rabbits. However, in two other teratogenicity studies, cleft palates developed in both rats and mice after administration of 5 to 9 times the human therapeutic dose of sulfisoxazole.
There is no evidence of teratogenicity or any other adverse effects on reproduction in female rats fed erythromycin base (up to 0.25% of diet) prior to and during mating, during gestation, and through weaning of two successive litters. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. Erythromycin has been reported to cross the placental barrier in humans, but fetal plasma levels are generally low.
There are no adequate or well-controlled studies of erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension in either laboratory animals or in pregnant women. It is not known whether erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension can cause fetal harm when administered to a pregnant woman prior to term or can affect reproduction capacity. Erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects:
Kernicterus may occur in the newborn as a result of treatment of a pregnant woman at term with sulfonamides. (See CONTRAINDICATIONS:.)
Labor and Delivery:
The effects of erythromycin and sulfisoxazole on labor and delivery are unknown.
Nursing Mothers:
Both erythromycin and sulfisoxazole are excreted in human milk. Because of the potential for the development of kernicterus in neonates due to the displacement of bilirubin from plasma proteins by sulfisoxazole, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother. (See CONTRAINDICATIONS:
Pediatric Use:
See INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION sections. Not for use in children under 2 months of age. (See CONTRAINDICATIONS.)
ADVERSE REACTIONS:
Erythromycin Ethylsuccinate:
The most frequent side effects of oral erythromycin preparations are gastrointestinal and are dose-related. They include nausea, vomiting, abdominal pain, diarrhea and anorexia. Symptoms of hepatic dysfunction and/or abnormal liver-function test results may occur (see WARNINGS section). Pseudomembranous colitis has been rarely reported in association with erythromycin therapy.
Allergic reactions ranging from urticaria and mild skin eruptions to anaphylaxis have occurred.
There have been isolated reports of reversible hearing loss occurring chiefly in patients with renal insufficiency and in patients receiving high doses of erythromycin.
Onset of pseudomembranous colitis symptoms may occur during of after antibiotic treatment. (See WARNINGS.)
Sulfisoxazole Acetyl:
Included in the listing that follows are adverse reactions that have been reported with other sulfonamide products: pharmacologic similarities require that each of the reactions be considered with erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension administration.
Allergic/Dermatologic:
Anaphylaxis, erythema multiforme (Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell’s syndrome), exfoliative dermatitis, angioedema, arteritis, vasculitis, allergic myocarditis, serum sickness, rash, urticaria, pruritus, photosensitivity, and conjunctival and scleral injection. In addition, periarteritis nodosa and systemic lupus erythematosus have been reported. (See WARNINGS.)
Cardiovascular:
Tachycardia, palpitations, syncope, and cyanosis.
Rarely, erythromycin has been associated with the production of ventricular arrhythmias, including ventricular tachycardia and torsade de pointes, in individuals with prolonged QT intervals.
Endocrine:
The sulfonamides bear certain chemical similarities to some goitrogens, diuretics (acetazolamide and the thiazides) and oral hypoglycemic agents. Cross-sensitivity may exist with these agents. Developments of goiter, diuresis, and hypoglycemia has occurred rarely in patients receiving sulfonamides.
Gastrointestinal:
Hepatitis, hepatocellular necrosis, jaundice, pseudomembranous colitis, nausea, emesis, anorexia, abdominal pain, diarrhea, gastrointestinal hemorrhage, melena, flatulence, glossitis, stomatitis, salivary gland enlargement, and pancreatitis. Onset of pseudomembranous colitis symptoms may occur during or after treatment with sulfisoxazole, a component of erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension. (See WARNINGS.)
-
Genitourinary:
Crystalluria, hematuria, BUN and creatinine elevations, nephritis, and toxic nephrosis with oliguria and anuria. Acute renal failure and urinary retention have also been reported.
-
Hematologic:
Leukopenia, agranulocytosis, aplastic anemia, thrombocytopenia, purpura, hemolytic anemia, anemia, eosinophilia, clotting disorders including hypopro-thrombinemia and hypofibrinogenemia, sulfhemoglobinemia, and methemoglobinemia.
Neurologic:
Headache, dizziness, peripheral neuritis, paresthesia, convulsions, tinnitus, vertigo, ataxia, and intracranial hypertension.
Psychiatric:
Psychosis, hallucinations, disorientation, depression, and anxiety.
Respiratory:
Cough, shortness of breath, and pulmonary infiltrates. (See WARNINGS)
Vascular:
Angioedema, arteritis, and vasculitis.
Miscellaneous:
Edema, (including periorbital), pyrexia, drowsiness, weakness, fatigue, lassitude, rigors, flushing, hearing loss, insomnia, and pneumonitis.
Overdosage:
No information is available on a specific result of overdose with erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension. Overdosage of erythromycin should be handled with the prompt elimination of unabsorbed drug and all other appropriate measures. Erythromycin is not removed by peritoneal dialysis or hemodialysis.
The amount of a single dose of sulfisoxazole that is either associated with symptoms of overdosage or is likely to be life-threatening has not been reported. Signs and symptoms of overdosage reported with sulfonamides include anorexia, colic, nausea, vomiting, dizziness, headache, drowsiness and unconsciousness. Pyrexia, hematuria and crystalluria may be noted. Blood dyscrasias and jaundice are potential late manifestations of overdosage.
General principles of treatment include the immediate discontinuation of the drug, instituting gastric lavage or emesis, forcing oral fluids, and administering intravenous fluids if urine output is low and renal function is normal. The patient should be monitored with blood counts and appropriate blood chemistries, including electrolytes. If the patient becomes cyanotic, the possibility of methemoglobinemia should be considered and, if present, the condition should be treated appropriately with intravenous 1% methylene blue. If a significant blood dyscrasia or jaundice occurs, specific therapy should be instituted for these complications. Peritoneal dialysis is not effective, and hemodialysis is only moderately effective in removing sulfonamides.
The acute toxicity of sulfisoxazole in animals is as follows:
|
Species |
LD50=S.E. (mg/kg) |
| mouse | 5700 = 235 |
| rats | ± 10,000 |
| rabbits | ± 2000 |
DOSAGE AND ADMINISTRATION:
ERYTHROMYCIN ETHYLSUCCINATE AND SULFISOXAZOLE ACETYL FOR ORAL SUSPENSION SHOULD NOT BE ADMINISTERED TO INFANTS UNDER 2 MONTHS OF AGE BECAUSE OF CONTRAINDICATIONS OF SYSTEMIC SULFONAMIDES IN THIS AGE GROUP.
For Acute Otitis Media in Children:
The dose of erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension can be calculated based on the erythromycin component (50 mg/kg/day) or the sulfisoxazole component (150 mg/kg/day to a maximum of 6 g/day). The total daily dose of erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension should be administered in equally divided doses three or four times a day for 10 days. Erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension may be administered without regard to meals.
The following approximate dosage schedules are recommended for using erythromycin ethylsuccinate and sulfisoxazole acetyl for oral suspension:
Children:
Two months of age or older
| FOUR-TIMES-A-DAY SCHEDULE | |
| Weight | Dose - every 6 hours |
| Less than 8 kg (<18 lbs) | Adjust dosage by body weight |
| 8 kg (18 lbs) | 1/2 teaspoonful (2.5 mL) |
| 16 kg (35 lbs) | 1 teaspoonful (5 mL) |
| 24 kg (53 lbs) | 1-1/2 teaspoonfuls (7.5 mL) |
| Over 32 kg (over 70 lbs) | 2 teaspoonfuls (10 mL) |
| THREE-TIMES-A-DAY SCHEDULE | |
| Weight | Dose - every 8 hours |
| Less than 6 kg (>13 lbs) | Adjust dosage by body weight |
| 6 kg (13 lbs) | 1/2 teaspoonful (2.5 mL) |
| 12 kg (26 lbs) | 1 teaspoonful (5 mL) |
| 18 kg (40 lbs) | 1-1/2 teaspoonfuls (7.5 mL) |
| 24 kg (53 lbs) | 2 teaspoonfuls (10 mL) |
| Over 30 kg (over 66 lbs) | 2-1/2 teaspoonfuls (12.5 mL) |
HOW SUPPLIED:
Erythromycin Ethylsuccinate and Sulfisoxazole Acetyl for Oral Suspension is available for teaspoon dosage in 100 mL (NDC 54868-0971-1) bottles, in the form of granules to be reconstituted with water.
The suspension provides erythromycin ethylsuccinate equivalent to 200 mg erythromycin activity and sulfisoxazole acetyl equivalent to 600 mg sulfisoxazole per teaspoonful (5 mL).
Store at room temperature in dry form.
REFERENCES:
- Biovert A, Barbeau G. Belanger PM: Pharmacokinetics of sulfisoxazole in young and elderly subjects. Gerontology 1984; 30: 125-131.
- Oie S. Gambertoglio JG, Fleckenstein L: Comparison of the disposition of total and unbound sulfisoxazole after single and multiple dosing. J Pharmacokinet Biopharm 1982; 10: 157 - 172.
- National Committee for Clinical Laboratory Standards: Performance Standards for Antimicrobial Disk Susceptibility Tests, ed 4. Approved Standard NCCLS Document M2-A4, Vol 10, No. 7. Villanova, Pa: NCCLS, 1990.
Manufactured by Duramed Pharmaceuticals, Inc.
Packaged by Pharmaceutics International, Inc.
Hunt Valley, Maryland 21031
for Duramed Pharmaceuticals, Inc.
Subsidiary of Barr Pharmaceuticals, Inc.
Pomona, NY 10970
Revised MARCH 2007
BR-0445
Relabeling of "Additional Barcode" label by:
Physicians Total Care, Inc.
Tulsa, OK 74146
PRINCIPAL DISPLAY PANEL

100 mL Label Text
E.S.P. (R)
Erythromycin Ethylsuccinate
and Sulfisoxazole Acetyl
for Oral Suspension, USP
200 mg erythromycin activity
and the equivalent of
600 mg sulfisoxazole
per 5 mL reconstituted
100 mL (when mixed)
Rx only
ERYTHROMYCIN ETHYLSUCCINATE AND SULFISOXAZOLE ACETYLERYTHROMYCIN ETHYLSUCCINATE AND SULFISOXAZOLE ACETYL GRANULE, FOR SUSPENSION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||