Escitalopram Oxalate
Camber Pharmaceuticals, Inc.
Hetero Labs Limited
HIGHLIGHTS OF PRESCRIBING INFORMATION BOXED WARNINGWARNING: Suicidality and Antidepressant Drugs See full prescribing information for complete boxed warning. Increased risk of suicidal thinking and behavior in children, adolescents and young adults taking antidepressants for major depressive disorder (MDD) and other psychiatric disorders. Escitalopram oxalate is not approved for use in pediatric patients less than 12 years of age (5.1).INDICATIONS AND USAGE1.11.2DOSAGE AND ADMINISTRATION2.12.2 Indication Recommended Dose MDD (2.1) Adolescents (2.1) Initial: 10 mg once dailyRecommended: 10 mg once dailyMaximum: 20 mg once daily Adults (2.1) Initial: 10 mg once dailyRecommended: 10 mg once daily Maximum: 20 mg once daily GAD (2.2) Adults (2.2) Initial: 10 mg once dailyRecommended: 10 mg once daily 2.12.32.32.4DOSAGE FORMS AND STRENGTHS3.2CONTRAINDICATIONS4.15.104.27.104.3WARNINGS AND PRECAUTIONS5.15.25.35.45.55.65.75.85.9Side Effects6.1To report SUSPECTED ADVERSE REACTIONS, contact Hetero Labs Limited at 866-495-1995, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS7.17.6USE IN SPECIFIC POPULATIONS8.18.38.4
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: Suicidality and Antidepressant Drugs
- 1 INDICATIONS & USAGE
- 2 DOSAGE & ADMINISTRATION
- 3 DOSAGE FORMS & STRENGTHS
- 4 ESCITALOPRAM OXALATE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 5.1 Clinical Worsening and Suicide Risk
- 5.2 Serotonin Syndrome or Neuroleptec Malignant Syndrome (NMS)-likeReactions
- 5.3 Discontinuation of Treatment with Escitalopram oxalate
- 5.4 Seizures
- 5.5 Activation of Mania/Hypomania
- 5.6 Hyponatremia
- 5.7 Abnormal Bleeding
- 5.8 Interference with Cognitive and Motor Performance
- 5.9 Use in Patients with Concomitant
- 5.10 Potential for Interaction with Monoamine Oxidase Inhibitors
- 6 ESCITALOPRAM OXALATE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 7.1 Serotonergic Drugs
- 7.2 Triptans
- 7.3 CNS Drugs
- 7.4 Alcohol
- 7.5 Monoamine Oxidase Inhibitors (MAOIs)
- 7.6 Drugs that Interfere With Hemostasis (NSAIDs, Aspirin, Warfarin, etc.)
- 7.7 Cimetidine
- 7.8 Digoxin
- 7.9 Lithium
- 7.10 Pimozide and Citalopram
- 7.11 Sumatriptan
- 7.12 Theophylline
- 7.13 Warfarin
- 7.14 Carbamazepine
- 7.15 Triazolam
- 7.16 Ketoconazole
- 7.17 Ritonavir
- 7.18 CYP3A4 and -2C19 Inhibitors
- 7.19 Drugs Metabolized by Cytochrome P4502D6
- 7.20 Metoprolol
- 8 USE IN SPECIFIC POPULATIONS
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 ESCITALOPRAM OXALATE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- MEDICATION GUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
WARNING: Suicidality and Antidepressant Drugs
WARNINGS: SUICIDALITY AND ANTIDEPRESSANT DRUGS
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of escitalopram oxalate or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Escitalopram oxalate is not approved for use in pediatric patients less than 12 years of age. [See Warnings and Precautions: Clinical Worsening and Suicide Risk (5.1), Patient Counseling Information: Information for Patients (17.1), and Used in Specific Populations: Pediatric Use (8.4)].
1 INDICATIONS & USAGE
1.1 Major Depressive Disorder
see Clinical Studies (14.1)
1.2 Generalized Anxiety Disorder
see Clinical Studies (14.2)
2 DOSAGE & ADMINISTRATION
2.1 Major Depressive Disorder
Initial Treatment
Adolescents
see Clinical Studies (14.1)
Adults
see Clinical Studies (14.1)
Maintenance Treatment
see Clinical Studies (14.1)
2.2 Generalized Anxiety Disorder
Initial Treatment
Adults
Maintenance Treatment
2.3 Special Populations
2.4 Discontinuation of Treatment with Escitalopram oxalate oral solution
see Warnings and Precautions (5.3)
2.5 Switching Patients To or From a Monoamine Oxidase Inhibitor
see Contraindications (4.1) and Warnings and Precautions (5.10)
3 DOSAGE FORMS & STRENGTHS
3.2 Oral Solution
4 CONTRAINDICATIONS
4.1 Monoamine oxidase inhibitors (MAOIs)
seeWarnings and Precautions (5.10)].
4.2 Pimozide
see Drug Interactions (7.10)
4.3 Hypersensitivity to escitalopram or citalopram
5 WARNINGS AND PRECAUTIONS
5.1 Clinical Worsening and Suicide Risk
| TABLE 1 |
|
| Age Range | Drug-Placebo Difference in Number of Cases of Suicidability per 1000 patients Treated |
|
Increases Compared to Placebo |
|
| <18 |
14 additional cases |
| 18-24 |
5 additional cases |
| Decreases Compared to Placebo |
|
| 25-64 |
1 fewer case |
|
≥65 |
6 fewer case |
see Dosage and Administration (2.4)
see also Patient Counseling Information (17.1)
Screening Patients for Bipolar Disorder
5.2 Serotonin Syndrome or Neuroleptec Malignant Syndrome (NMS)-likeReactions
5.3 Discontinuation of Treatment with Escitalopram oxalate
see Dosage and Administration (2.4)
5.4 Seizures
5.5 Activation of Mania/Hypomania
5.6 Hyponatremia
see Geriatric Use (8.5)
5.7 Abnormal Bleeding
5.8 Interference with Cognitive and Motor Performance
5.9 Use in Patients with Concomitant
see Dosage and Administration (2.3)
see Dosage and Administration (2.3)
5.10 Potential for Interaction with Monoamine Oxidase Inhibitors
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Clinical Trial Data Sources
Pediatrics (6 to 17 years)
Adults
Adverse Events Associated with Discontinuation of Treatment
Major Depressive disorder
Pediatrics (6 to 17 years)
Adults
Generalized Anxiety Disorder
Adults
Incidence of Adverse Reactions in Placebo-Controlled Clinical Trials
Major Depressive disorder
Pediatrics (6 to 17 years)
Adults
|
TABLE 2
|
||
|
Treatment-Emergent Adverse Reactions observed with a frequency of ≥ 2% and greater than placebo for Major Depressive Disorder
|
||
|
Adverse Reaction
|
Escitalopram oxalate
|
Placebo
|
| |
|
|
|
|
(N=715)
% |
(N=592)
% |
|
Autonomic Nervous System Disorders
|
|
|
| Dry Mouth |
6% |
5% |
| Sweating Increased |
5% |
2% |
|
Central & Peripheral Nervous System Disorders
|
||
| Dizziness |
5% |
3% |
|
Gastrointestinal Disorders
|
|
|
| Nausea |
15% |
7% |
| Diarrhea |
8% |
5% |
| Constipation |
3% |
1% |
| Indigestion |
3% |
1% |
| Abdominal Pain |
2% |
1% |
|
General
|
|
|
| Influenza-like Symptoms |
5% |
4% |
| Fatigue |
5% |
2% |
|
Psychiatric Disorders
|
|
|
| Insomnia |
9% |
4% |
| Somnolence |
6% |
2% |
| Apetite Decreased |
3% |
1% |
| Libido Decreased |
3% |
1% |
|
Respiratory System Disorders
|
|
|
| Rhinitis |
5% |
4% |
| Sinustis |
3% |
2% |
|
Urogenital
|
|
|
|
Ejaculation Disorder1,2
|
9% |
<1% |
| Impotence2
|
3% |
<1% |
| Anorgasmia3
|
2% |
<1% |
1
2
3
Generalized Anxiety Disorder
Adults
Table 3
|
TABLE 3
|
|||
|
Treatment-Emergent Adverse Reactions observed with a frequency of ≥ 2% and greater than placebo for Generalized Anxiety Disorder
|
|||
|
Adverse Reactions
|
Escitalopram
oxalate
|
Placebo
|
|
| |
|
|
|
|
|
(N=429)
% |
(N=427)
% |
|
|
Autonomic Nervous System Disorders
|
|
|
|
| Dry Mouth |
9% |
5% |
|
| Sweating Increased |
4% |
1% |
|
|
Central & Peripheral Nervous System Disorders
|
|||
| Headache |
24% |
17% |
|
| Paresthesia |
2% |
1% |
|
|
Gastrointestinal Disorders
|
|
|
|
| Nausea |
18% |
8% |
|
| Diarrhea |
8% |
6% |
|
| Constipation |
5% |
4% |
|
| Indigestion |
3% |
2% |
|
| Vomiting |
3% |
1% |
|
| Abdominal Pain |
2% |
1% |
|
| Flatulence |
2% |
1% |
|
| Toothache |
2% |
0% |
|
|
General
|
|
|
|
| Fatigue |
8% |
2% |
|
| Influenza-like Symptoms |
5% |
4% |
|
| Musculoskeletal System Disorder
|
|
|
|
| Neck/Shoulder Pain |
3% |
1% |
|
| Psychiatric Disorders
|
|
|
|
| Somnolence |
13% |
7% |
|
| Insomnia |
12% |
6% |
|
| Libido Decreased |
7% |
2% |
|
| Dreaming Abnormal |
3% |
2% |
|
| Appetite Decreased |
3% |
1% |
|
| Lethargy |
3% |
1% |
|
|
Respiratory System Disorders
|
|
|
|
| Yawning |
2% |
1% |
|
|
Urogenital
|
|
|
|
|
Ejaculation Disorder1,2
|
14% |
2% |
|
|
Anorgasmia3
|
6% |
<1% |
|
|
Menstrual Disorder |
2% |
1% |
|
1
2
3
Dose Dependency of Adverse Reactions
| TABLE 4
|
|||
|
Incidence of Common Adverse Reactions in Patients with
Major Depressive Disorder |
|||
|
Adverse Reaction
|
Placebo
|
10 mg/day
|
20 mg/day
|
| |
(N=311)
|
Escitalopram
oxalate
|
Escitalopram
oxalate
|
|
|
|
(N=310)
|
(N=125)
|
|
Insomnia
|
4% |
7% |
14% |
|
Diarrhea
|
5% |
6% |
14% |
|
Dry Mouth
|
3% |
4% |
9% |
|
Somnolence
|
1% |
4% |
9% |
|
Dizziness
|
2% |
4% |
7% |
|
Sweating Increased
|
<1% |
3% |
8% |
|
Constipation
|
1% |
3% |
6% |
|
Fatigue
|
2% |
2% |
6% |
|
Indigestion
|
1% |
2% |
6% |
Male and Female Sexual Dysfunction with SSRIs
Although changes in sexual desire, sexual performance, and sexual satisfaction often occur as manifestations of a psychiatric disorder, they may also be a consequence of pharmacologic treatment. In particular, some evidence suggests that SSRIs can cause such untoward sexual experiences.
|
TABLE 5 |
|||
|
Incidence of Sexual Side Effects in Placebo-Controlled Clinical Trials |
|||
|
Adverse Event |
Escitalopram oxalate |
Placebo |
|
|
|
In Males Only |
||
|
|
(N=407) |
(N=383) |
|
|
Ejaculation Disorder (primarily ejaculatory delay) |
12% |
1% |
|
|
Libido Decreased |
6% |
2% |
|
|
Impotence |
2% |
<1% |
|
|
|
In Females Only |
||
|
|
(N=737) |
(N=636) |
|
|
Libido Decreased |
3% |
1% |
|
|
Anorgasmia |
3% |
<1% |
|
Vital Sign Changes
Weight Changes
Laboratory Changes
ECG Changes
Other Reactions Observed During the Premarketing Evaluation of Escitalopram oxalate
ADVERSE REACTIONS
6.2 Post-Marketing Experience
Adverse Reactions Reported Subsequent to the Marketing of Escitalopram
7 DRUG INTERACTIONS
7.1 Serotonergic Drugs
see Warnings and Precautions (5.2)
7.2 Triptans
see Warnings and Precautions (5.2)
7.3 CNS Drugs
7.4 Alcohol
7.5 Monoamine Oxidase Inhibitors (MAOIs)
see Contraindications (4.1) and Warnings and Precautions (5.10)
7.6 Drugs that Interfere With Hemostasis (NSAIDs, Aspirin, Warfarin, etc.)
7.7 Cimetidine
max
7.8 Digoxin
7.9 Lithium
7.10 Pimozide and Citalopram
max
7.11 Sumatriptan
There have been rare postmarketing reports describing patients with weakness, hyperreflexia, and incoordination following the use of an SSRI and sumatriptan. If concomitant treatment with sumatriptan and an SSRI (e.g., fluoxetine, fluvoxamine, paroxetine, sertraline, citalopram, escitalopram) is clinically warranted, appropriate observation of the patient is advised.
7.12 Theophylline
7.13 Warfarin
7.14 Carbamazepine
7.15 Triazolam
7.16 Ketoconazole
max
7.17 Ritonavir
7.18 CYP3A4 and -2C19 Inhibitors
In vitro
7.19 Drugs Metabolized by Cytochrome P4502D6
In vitroin vivomax
7.20 Metoprolol
max
7.21 Electroconvulsive Therapy (ECT)
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects:
see Warnings and Precautions (5.2)
see Dosage and Administration (2.1)
8.2 Labor & Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
Clinical Studies (14.1)
8.5 Geriatric Use
see Hyponatremia (5.6)
see Clinical Pharmacology (12.3) see Dosage and Administration (2.3)
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse and Dependence
10 OVERDOSAGE
10.1 Human Experience
10.2 Management of Overdose
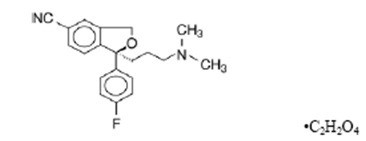
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
In vitroin vivo
12.3 Pharmacokinetics
The single- and multiple-dose pharmacokinetics of escitalopram are linear and dose-proportional in a dose range of 10 to 30 mg/day. Biotransformation of escitalopram is mainly hepatic, with a mean terminal half-life of about 27 to 32 hours. With once-daily dosing, steady state plasma concentrations are achieved within approximately one week. At steady state, the extent of accumulation of escitalopram in plasma in young healthy subjects was 2.2 to 2.5 times the plasma concentrations observed after a single dose. The tablet and the oral solution dosage forms of escitalopram oxalate are bioequivalent.
Absorption and Distribution
Following a single oral dose (20 mg tablet or solution) of escitalopram, peak blood levels occur at about 5 hours. Absorption of escitalopram is not affected by food.
The absolute bioavailability of citalopram is about 80% relative to an intravenous dose, and the volume of distribution of citalopram is about 12 L/kg. Data specific on escitalopram are unavailable.
The binding of escitalopram to human plasma proteins is approximately 56%.
Metabolism and Elimination
Following oral administrations of escitalopram, the fraction of drug recovered in the urine as escitalopram and S-demethylcitalopram (S-DCT) is about 8% and 10%, respectively. The oral clearance of escitalopram is 600 mL/min, with approximately 7% of that due to renal clearance.
Escitalopram is metabolized to S-DCT and S-didemethylcitalopram (S-DDCT). In humans, unchanged escitalopram is the predominant compound in plasma. At steady state, the concentration of the escitalopram metabolite S-DCT in plasma is approximately one-third that of escitalopram. The level of S-DDCT was not detectable in most subjects. In vitro studies show that escitalopram is at least 7 and 27 times more potent than S-DCT and S-DDCT, respectively, in the inhibition of serotonin reuptake, suggesting that the metabolites of escitalopram do not contribute significantly to the antidepressant actions of escitalopram. S-DCT and S-DDCT also have no or very low affinity for serotonergic (5-HT1 to 7) or other receptors including alpha- and beta-adrenergic, dopamine (D1 to 5), histamine (H1 to 3), muscarinic (M1 to 5), and benzodiazepine receptors. S-DCT and S-DDCT also do not bind to various ion channels including Na+, K+, Cl-, and Ca++ channels.
In vitro studies using human liver microsomes indicated that CYP3A4 and CYP2C19 are the primary isozymes involved in the N-demethylation of escitalopram.
Population Subgroups
Age
Adolescents - In a single dose study of 10 mg escitalopram, AUC of escitalopram decreased by 19%, and Cmax increased by 26% in healthy adolescent subjects (12 to 17 years of age) compared to adults. Following multiple dosing of 40 mg/day citalopram, escitalopram elimination half-life, steady-state Cmax and AUC were similar in patients with MDD (12 to 17 years of age) compared to adult patients. No adjustment of dosage is needed in adolescent patients.
Elderly - Escitalopram pharmacokinetics in subjects ≥ 65 years of age were compared to younger subjects in a single-dose and a multiple-dose study. Escitalopram AUC and half-life were increased by approximately 50% in elderly subjects, and Cmax was unchanged. 10 mg is the recommended dose for elderly patients [see Dosage and Administration (2.3) ].
Gender - Based on data from single- and multiple-dose studies measuring escitalopram in elderly, young adults, and adolescents, no dosage adjustment on the basis of gender is needed.
Reduced hepatic function - Citalopram oral clearance was reduced by 37% and half life was doubled in patients with reduced hepatic function compared to normal subjects. 10 mg is the recommended dose of escitalopram for most hepatically impaired patients [see Dosage and Administration (2.3) ].
Reduced renal function - In patients with mild to moderate renal function impairment, oral clearance of citalopram was reduced by 17% compared to normal subjects. No adjustment of dosage for such patients is recommended. No information is available about the pharmacokinetics of escitalopram in patients with severely reduced renal function (creatinine clearance < 20 mL/min).
Drug-Drug Interactions
In vitro in vitro in vivo in vivo Drug Interactions (7.18)
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
in vitroin vitroin vitro/in vivo in vitro
13.2 Animal Pharmacology & OR Toxicology
14 CLINICAL STUDIES
14.1 Major Depressive Disorder
Adolescents
Adults
14.2 Generalized Anxiety Disorder
16 HOW SUPPLIED/STORAGE AND HANDLING
16.2 Oral Solution
Store at 20 to 25°C (68 to 77°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
17.1 Information for Patients
General Information about Medication Guide
Clinical Worsening and Suicide Risk
see Warnings and Precautions (5.1)
Serotonin Syndrome
see Warnings and Precautions (5.2)
Abnormal Bleeding
see Warnings and Precautions (5.7)
Concomitant Medications
Continuing the Therapy Prescribed
Interference with Psychomotor Performance
Alcohol
Pregnancy and Breast Feeding
Need for Comprehensive Treatment Program
17.2 FDA-Approved Medication Guide
Escitalopram Oxalate Oral Solution
MEDICATION GUIDE
What is the most important information I should know about escitalopram oxalate oral solution?
1. Suicidal thoughts or actions:
scitalopram oxalate oral solution and other antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, or young adults within the first few months of treatment or when the dose is changed.
Call your healthcare provider right away if you have any of the following symptoms, or call 911 if an emergency, especially if they are new, worse, or worry you:
Call your healthcare provider right away if you have any of the following symptoms, or call 911 if an emergency. Escitalopram oxalate oral solution may be associated with these serious side effects:
2. Serotonin Syndrome or Neuroleptic Malignant Syndrome-like reactions. This condition can be life-threatening and may include:
3. Severe allergic reactions:
4. Abnormal bleeding:
5. Seizures or convulsions
6. Manic episodes:
7. Changes in appetite or weight.
8. Low salt (sodium) levels in the blood. Elderly people may be at greater risk for this. Symptoms may include:
Do not stop escitalopram oxalate oral solution without first talking to your healthcare provider. Stopping escitalopram oxalate oral solution too quickly may cause serious symptoms including:
What is escitalopram oxalate oral solution?
Who should not take escitalopram oxalate oral solution?
People who take escitalopram oxalate oral solution close in time to an MAOI may have serious or even life-threatening side effects. Get medical help right away if you have any of these symptoms:
take the antipsychotic medicine pimozide (Orap®) because taking this drug with escitalopram oxalate oral solution can cause serious heart problems.
What should I tell my healthcare provider before taking escitalopram oxalate oral solution? Ask if you are not sure.
Tell your healthcare provider about all thethat you take
|
If you take escitalopram oxalate oral solution, you should not take any other medicines that contain escitalopram oxalate or citalopram hydrobromide including: Citalopram. |
How should I take escitalopram oxalate oral solution?
What should I avoid while taking escitalopram oxalate oral solution?
What are the possible side effects of escitalopram oxalate oral solution?
CALL YOUR DOCTOR FOR MEDICAL ADVICE ABOUT SIDE EFFECTS. YOU MAY REPORT SIDE EFFECTS TO THE FDA AT 1-800-FDA-1088.
How should I store escitalopram oxalate oral solution?
Keep escitalopram oxalate oral solution and all medicines out of the reach of children.
General information about escitalopram oxalate oral solution
What are the ingredients in escitalopram oxalate oral solution?
®®®
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

Escitalopram OxalateEscitalopram Oxalate SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||