Ventura Corporation LTD
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
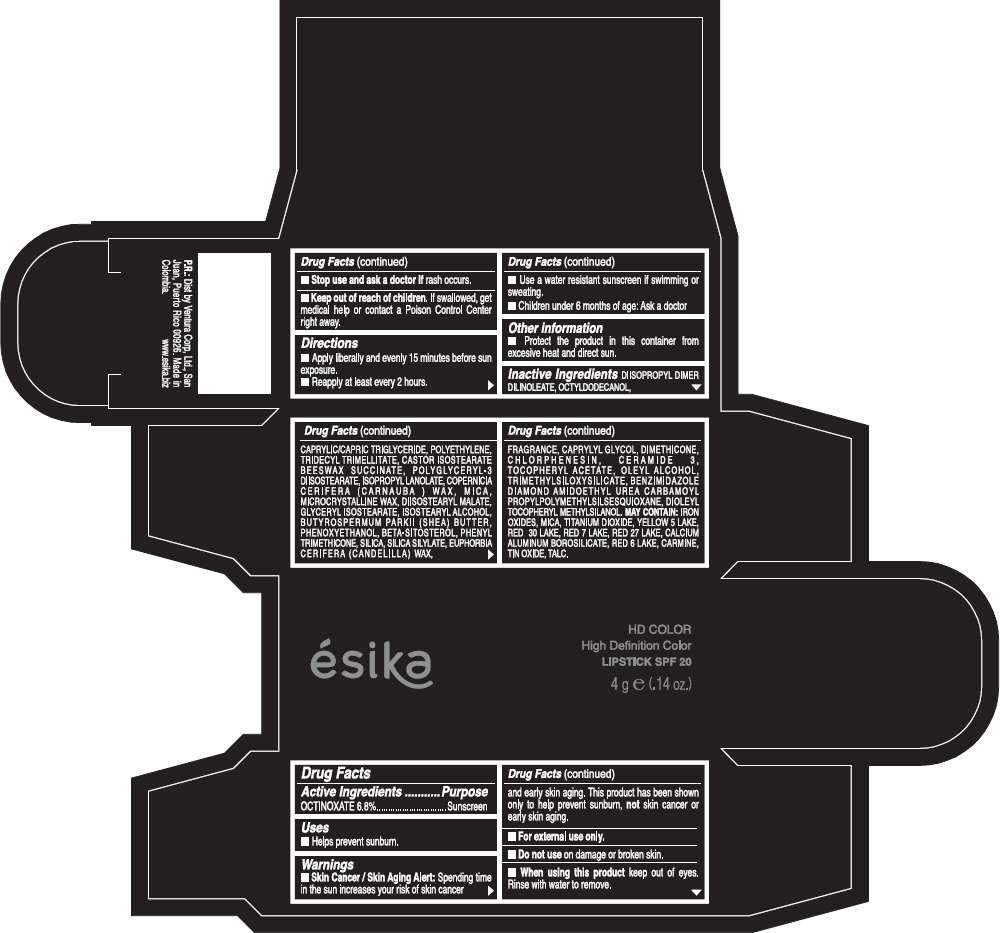
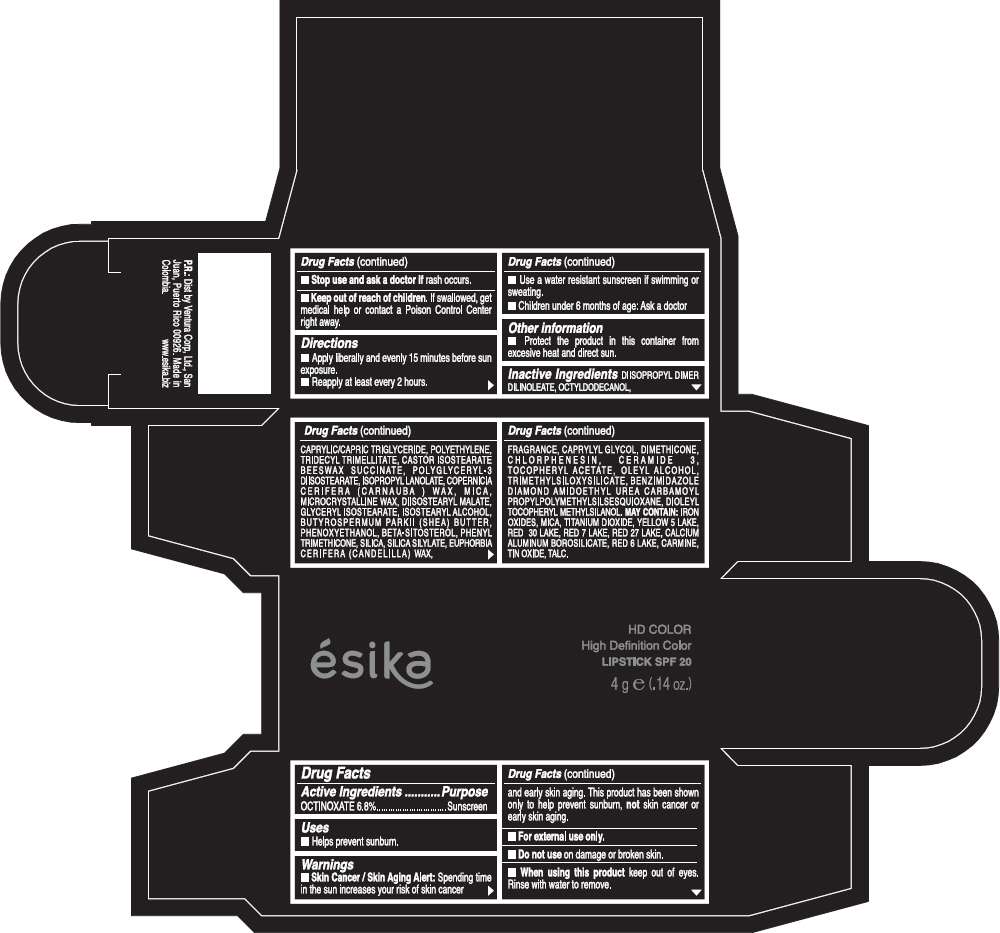
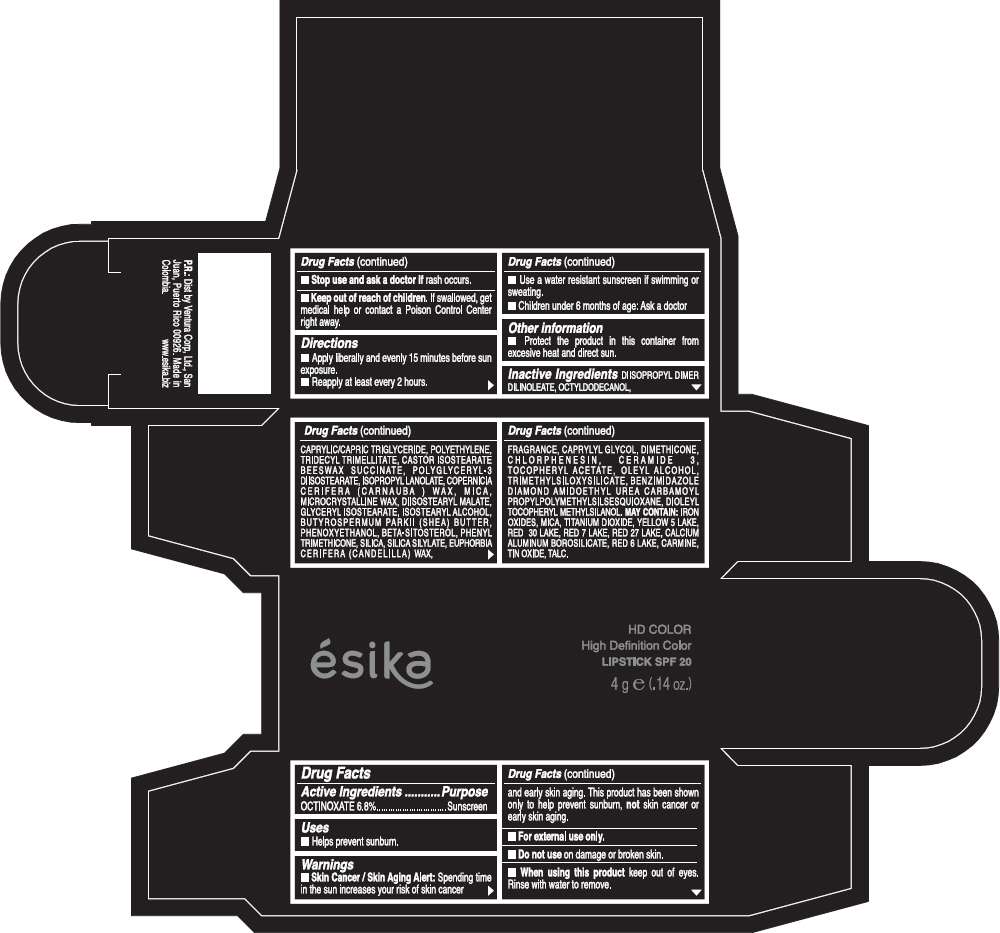
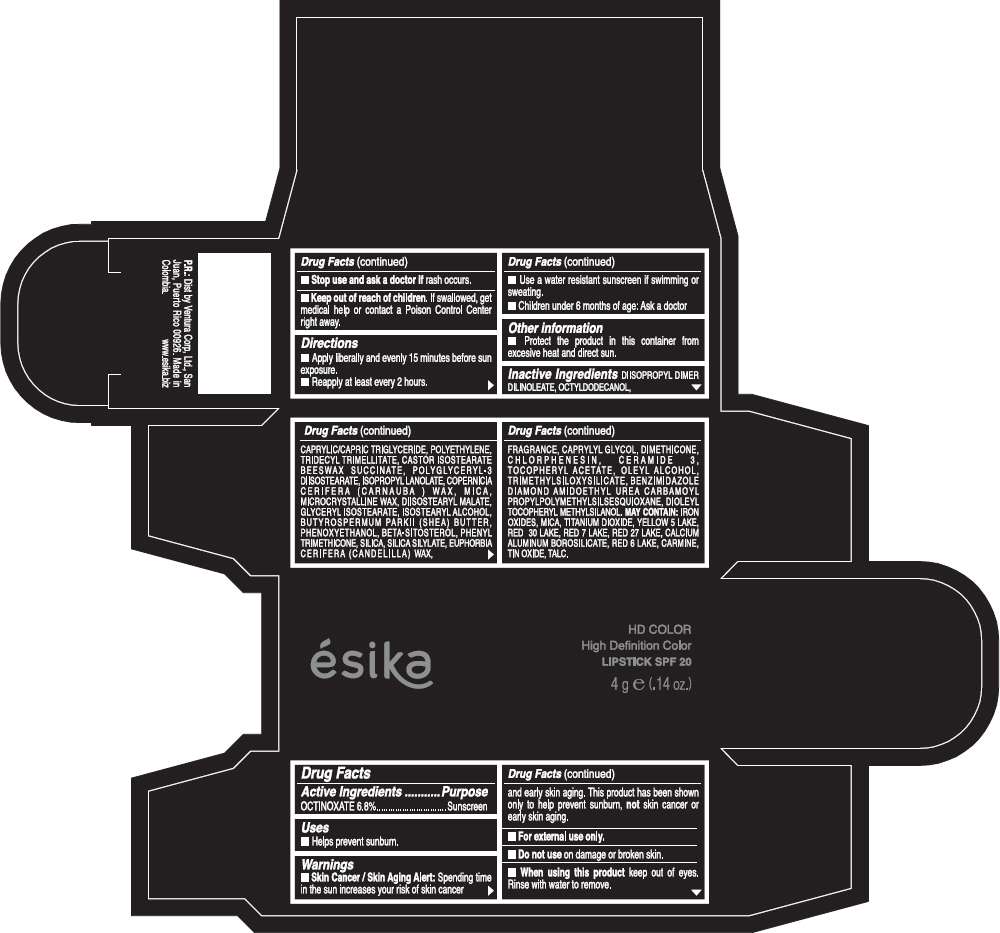
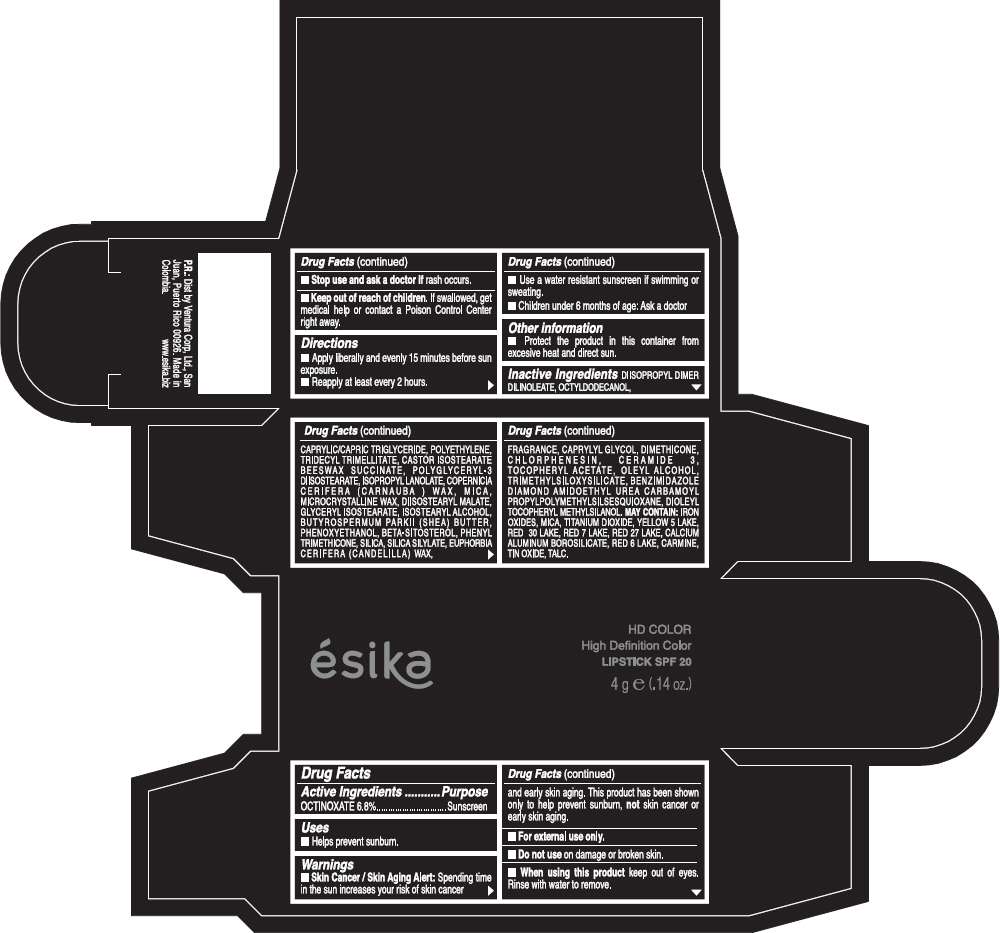
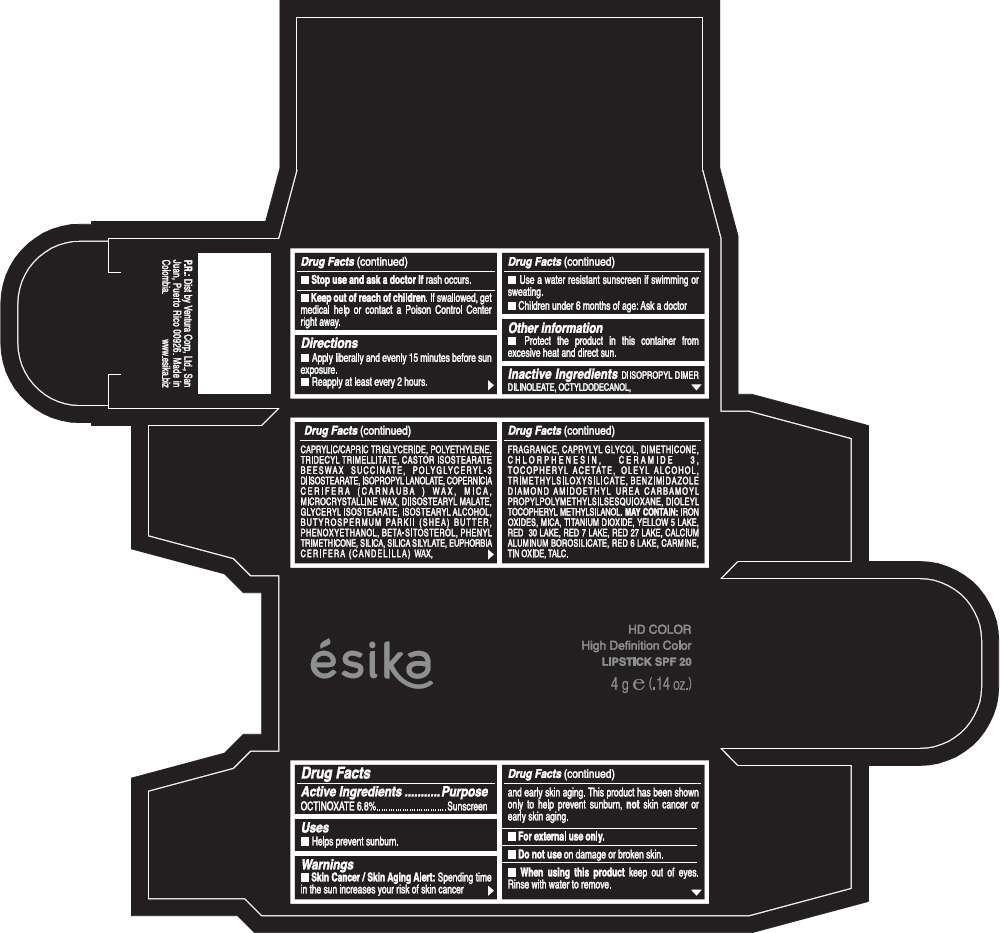
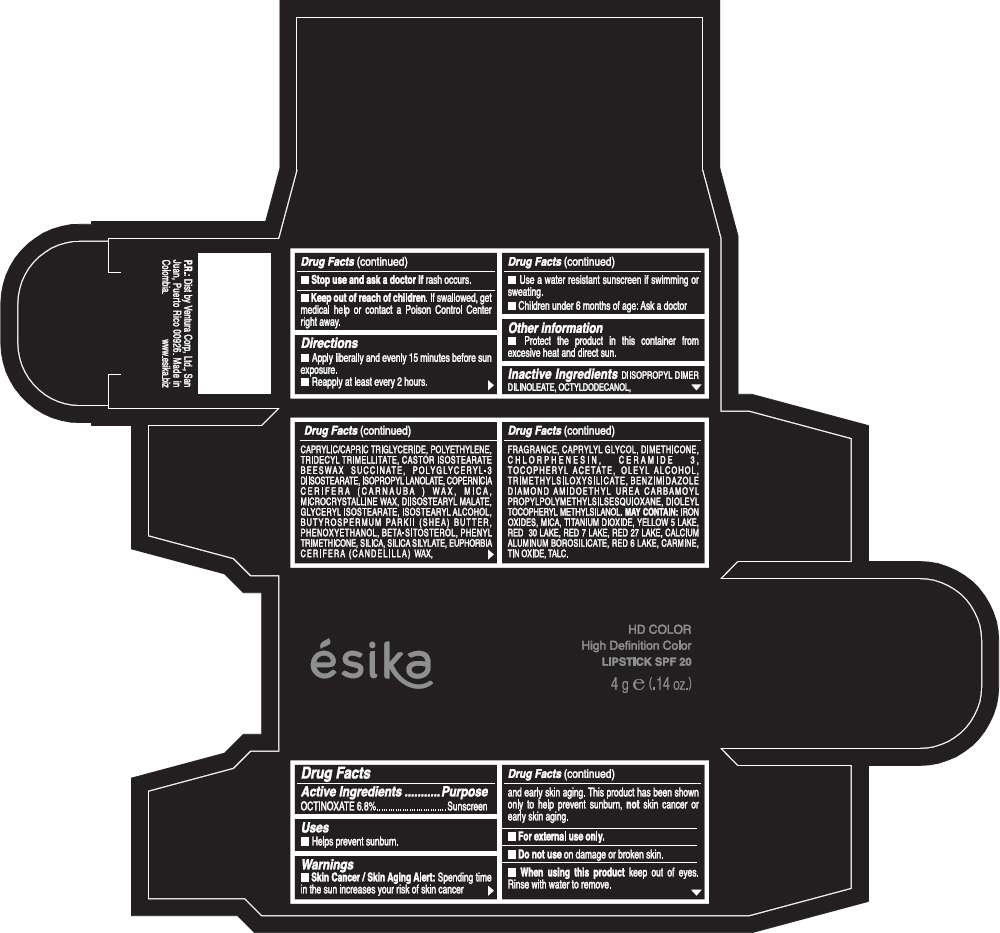
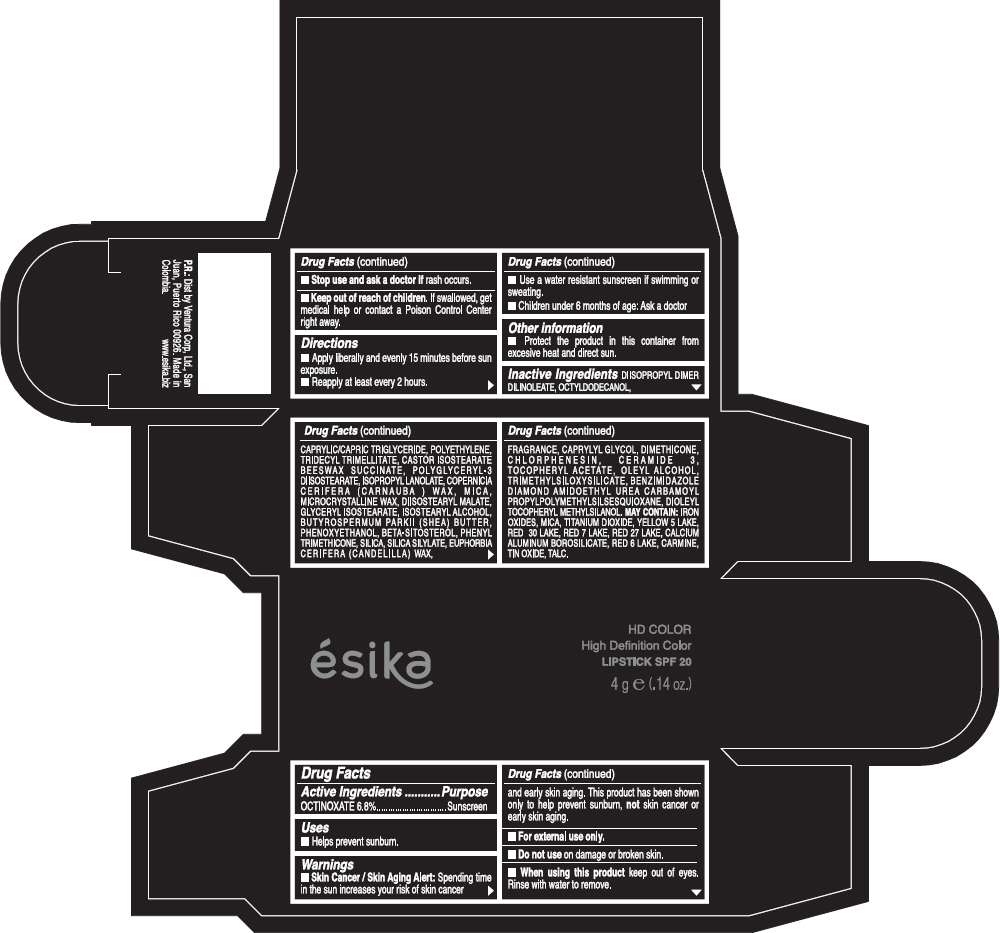
Drug Facts
Active Ingredients
OCTINOXATE (6.8%)
Purpose
Sunscreen
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 Uses
Warnings
-
Skin Cancer / Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
-
For external use only.
-
Do not use on damaged or broken skin.
-
When using this product keep out of eyes.Rinse with water to remove.
-
Stop use and ask a doctor if rash occurs.
-
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally and evenly 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Children under 6 months of age: Ask a doctor
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20 Other information
- Protect this product in this container form excesive heat and direct sun.
Inactive ingredients
DIISOPROPYL DIMER DILINOLEATE, OCTYLDODECANOL, CAPRYLIC/CAPRIC TRIGLYCERIDE, POLYETHYLENE, TRIDECYL TRIMELLITATE, CASTOR ISOSTEARATE BEESWAX SUCCINATE, POLYGLYCERYL-3 DIISOSTEARATE, ISOPROPYL LANOLATE, COPERNICIA CERIFERA (CARNAUBA ) WAX, MICA, MICROCRYSTALLINE WAX, DIISOSTEARYL MALATE, GLYCERYL ISOSTEARATE, ISOSTEARYL ALCOHOL, BUTYROSPERMUM PARKII (SHEA) BUTTER, PHENOXYETHANOL, BETA-SITOSTEROL, PHENYL TRIMETHICONE, SILICA, SILICA SILYLATE, EUPHORBIA CERIFERA (CANDELILLA) WAX, FRAGRANCE, CAPRYLYL GLYCOL, DIMETHICONE, CHLORPHENESIN, CERAMIDE 3, TOCOPHERYL ACETATE, OLEYL ALCOHOL, TRIMETHYLSILOXYSILICATE, BENZIMIDAZOLE DIAMOND AMIDOETHYL UREA CARBAMOYL PROPYL POLYMETHYLSILSESQUIOXANE, DIOLEYL TOCOPHERYL METHYLSILANOL.
May contain:
IRON OXIDES, MICA, TITANIUM DIOXIDE, YELLOW 5 LAKE, RED 30 LAKE, RED 7 LAKE, RED 27 LAKE, CALCIUM ALUMINUM BOROSILICATE, RED 6 LAKE, CARMINE, TIN OXIDE, TALC
P.R.: Dist by Ventura Corp, Ltd., San
Juan, Puerto Rico 00926.
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD MARRÓN TOSCANA) - BROWN
ésika
HD COLOR
High Definition Color
LIPSTICK SPF 20
4 g e (.14 oz.)
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD PIMIENTA CALIENTE) - BROWN
ésika
HD COLOR
High Definition Color
LIPSTICK SPF 20
4 g e (.14 oz.)
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD VINO CAUTIVANTE) - RED
ésika
HD COLOR
High Definition Color
LIPSTICK SPF 20
4 g e (.14 oz.)
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD ROSA VIVA) - PINK
ésika
HD COLOR
High Definition Color
LIPSTICK SPF 20
4 g e (.14 oz.)
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD CORAL ENSUEÑO) - RED
ésika
HD COLOR
High Definition Color
LIPSTICK SPF 20
4 g e (.14 oz.)
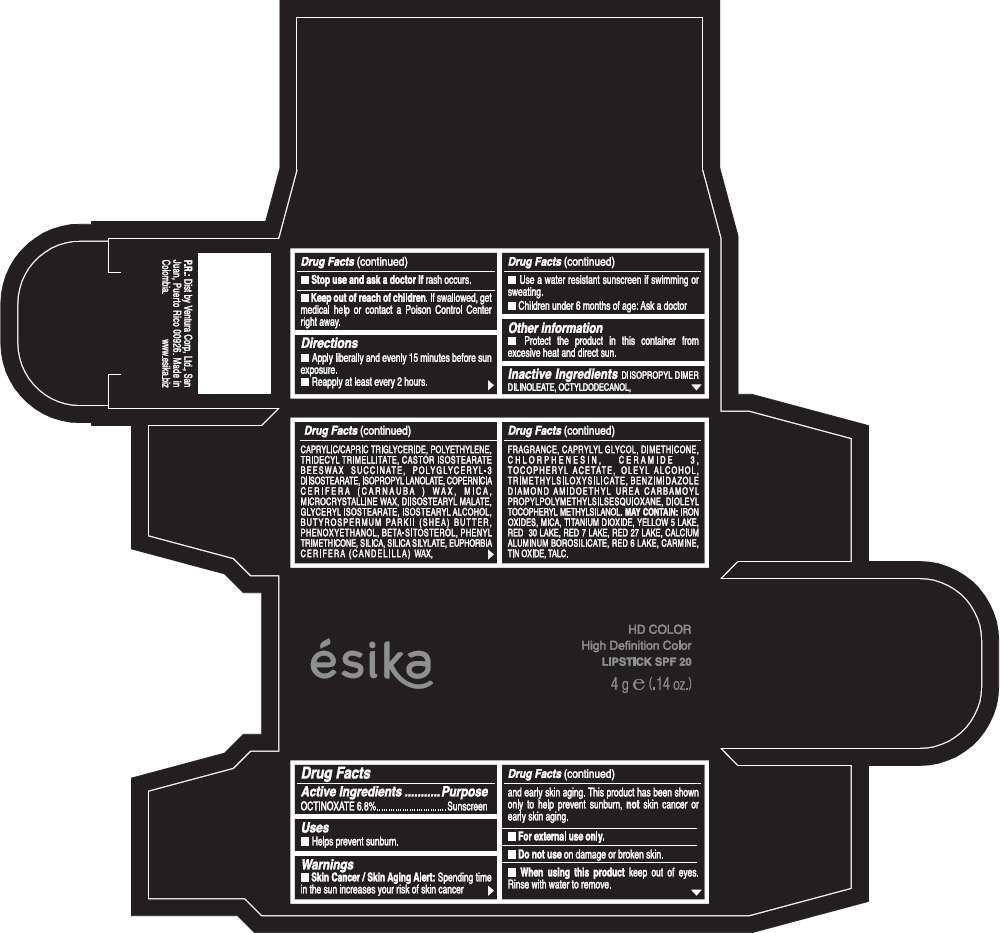
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD ROJO GLAM) - RED
ésika
HD COLOR
High Definition Color
LIPSTICK SPF 20
4 g e (.14 oz.)
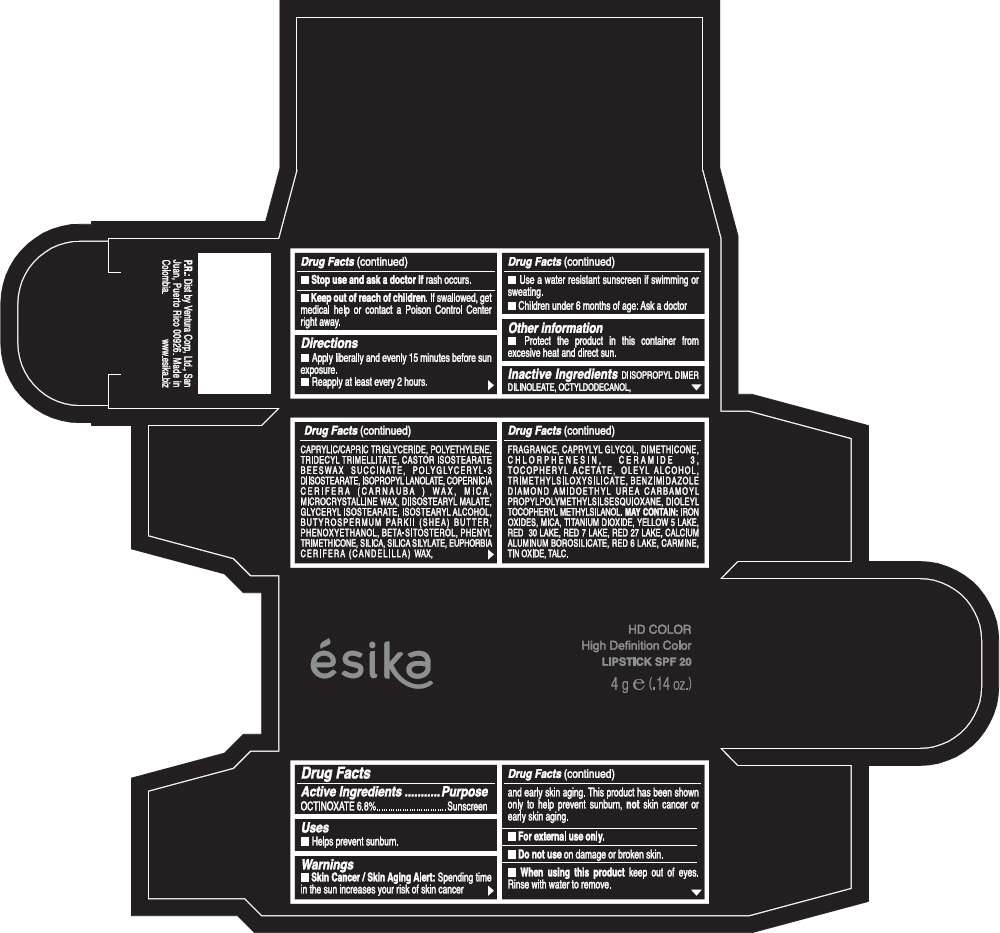
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD CAFÉ HABANO) - BROWN
ésika
HD COLOR
High Definition Color
LIPSTICK SPF 20
4 g e (.14 oz.)
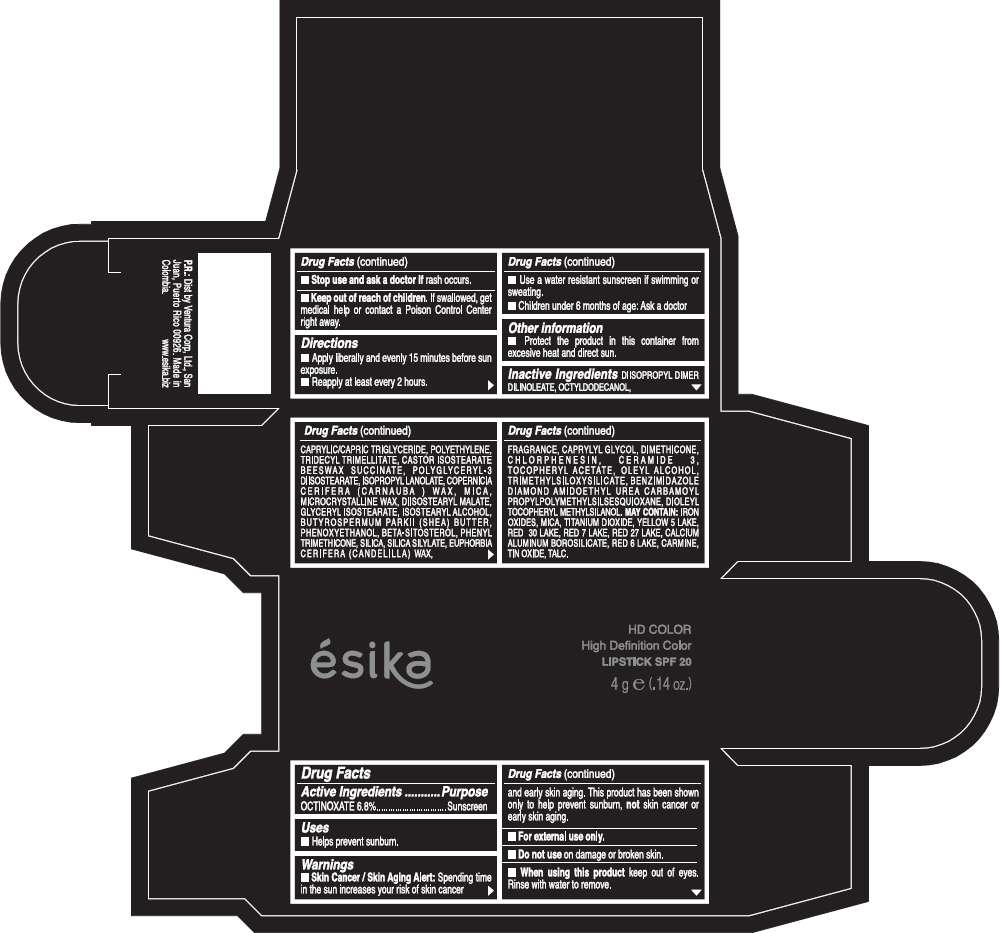
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD FUCSIA VIBRANTE) - PINK
ésika
HD COLOR
High Definition Color
LIPSTICK SPF 20
4 g e (.14 oz.)
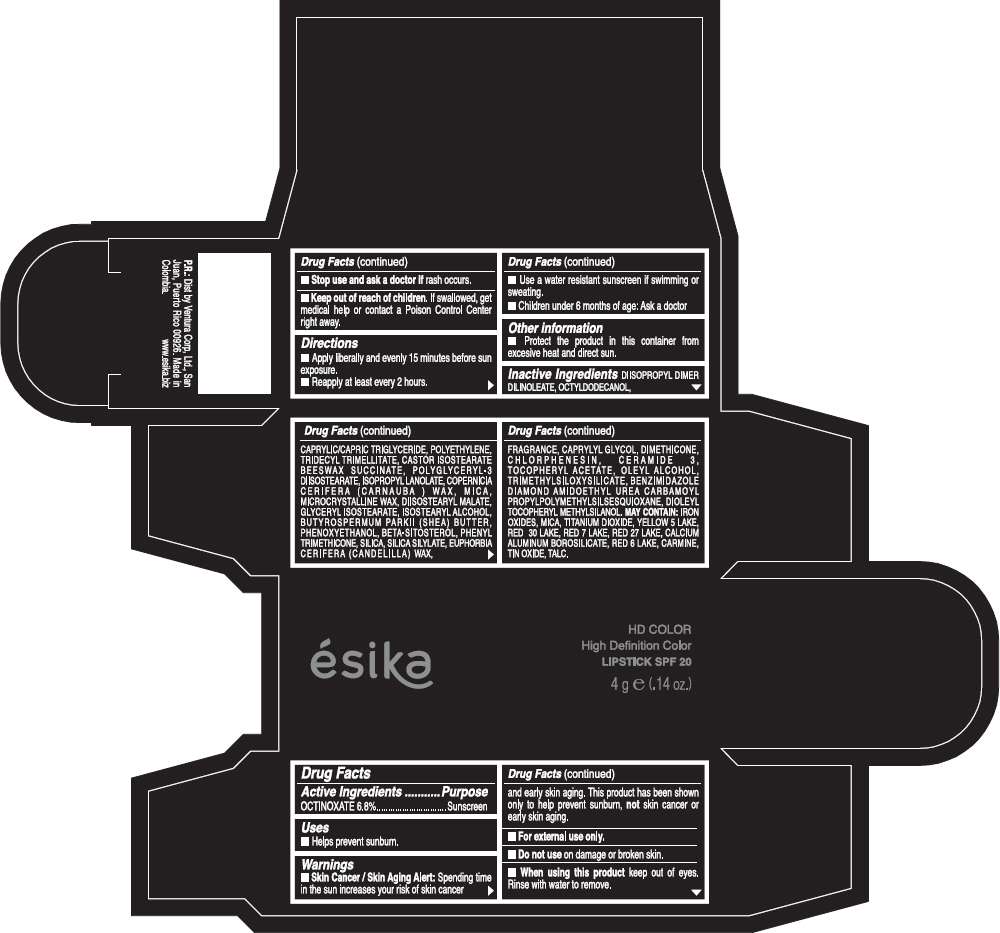
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD DURAZNO NUDE) - PINK
ésika
HD COLOR
High Definition Color
LIPSTICK SPF 20
4 g e (.14 oz.)

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD NARANJA PROVOCACIÓN) - ORANGE
ésika
HD COLOR
High Definition Color
LIPSTICK SPF 20
4 g e (.14 oz.)
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD MELÓN INTENSE) - PINK
ésika
HD COLOR
High Definition Color
LIPSTICK SPF 20
4 g e (.14 oz.)
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD ROSADO CHIC) - PINK
ésika
HD COLOR
High Definition Color
LIPSTICK SPF 20
4 g e (.14 oz.)
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD CIRUELA GLAM) - RED
ésika
HD COLOR
High Definition Color
LIPSTICK SPF 20
4 g e (.14 oz.)
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD FRAMBUESA ENCANTO) - RED
ésika
HD COLOR
High Definition Color
LIPSTICK SPF 20
4 g e (.14 oz.)
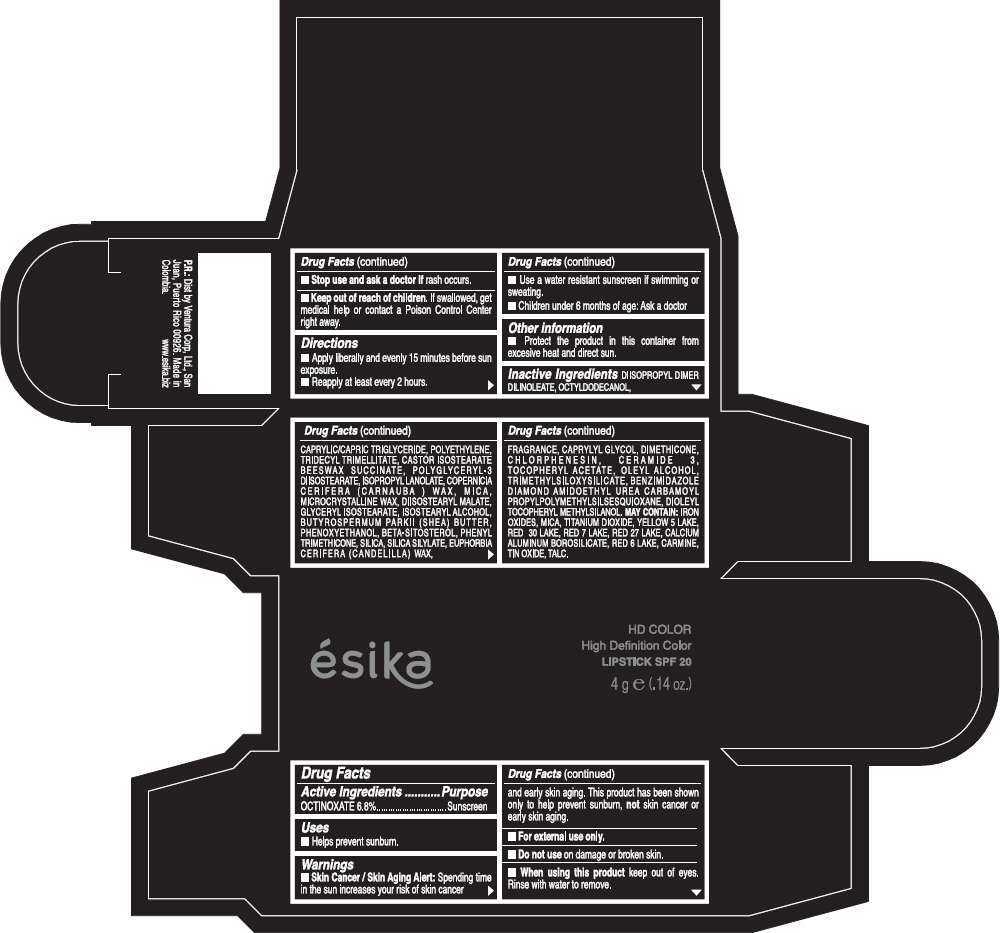
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD MAGENTA SWEET) - PURPLE
ésika
HD COLOR
High Definition Color
LIPSTICK SPF 20
4 g e (.14 oz.)
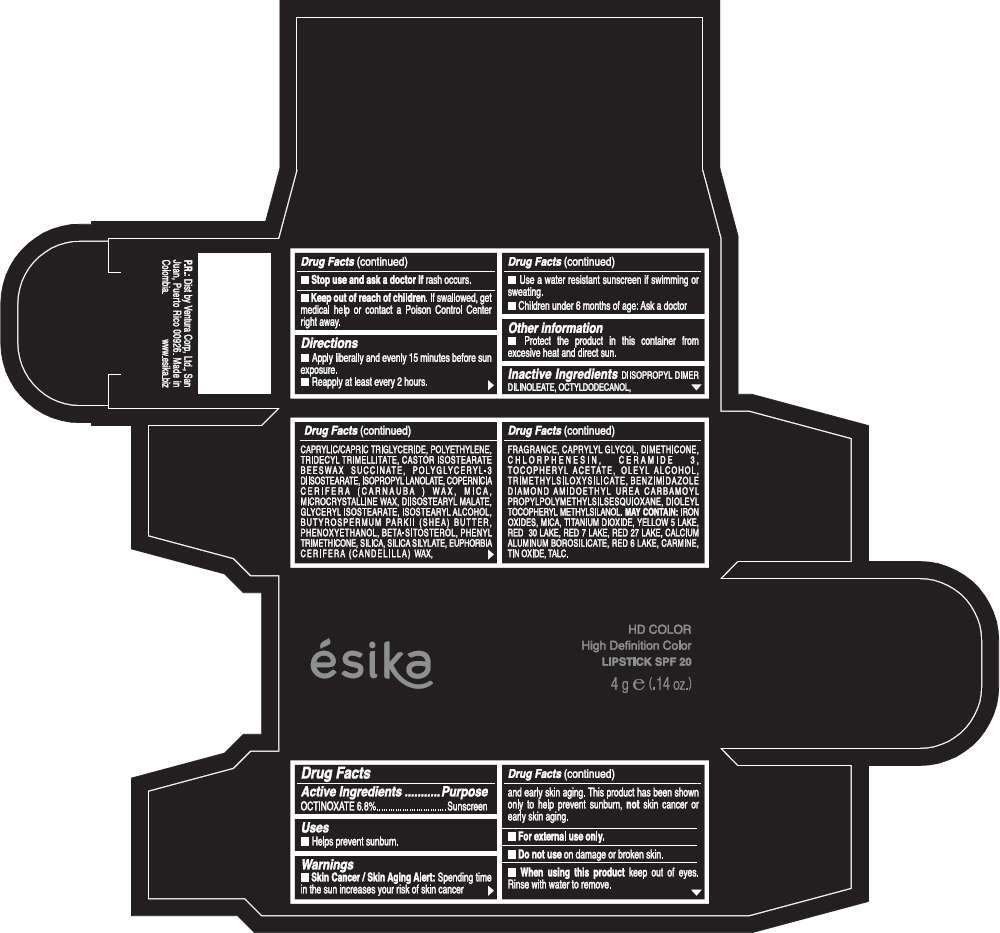
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20
Octinoxate LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-001 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.068 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-001-01 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-001-02 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-04-03 |
|
|
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20
Octinoxate LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-002 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.068 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-002-03 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-002-04 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-04-03 |
|
|
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20
Octinoxate LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-003 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.068 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-003-05 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-003-06 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-04-03 |
|
|
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20
Octinoxate LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-004 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.068 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-004-07 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-004-08 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-04-03 |
|
|
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20
Octinoxate LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-005 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.068 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-005-09 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-005-10 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-04-03 |
|
|
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20
Octinoxate LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-006 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.068 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-006-11 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-006-12 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-04-03 |
|
|
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20
Octinoxate LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-007 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.068 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-007-13 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-007-14 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-04-03 |
|
|
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20
Octinoxate LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-008 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.068 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-008-15 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-008-16 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-04-03 |
|
|
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20
Octinoxate LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-009 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.068 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-009-17 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-009-18 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-04-03 |
|
|
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20
Octinoxate LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-016 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.068 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-016-19 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-016-20 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-04-03 |
|
|
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20
Octinoxate LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-017 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.068 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-017-21 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-017-22 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-04-03 |
|
|
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20
Octinoxate LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-018 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.068 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-018-23 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-018-24 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-04-03 |
|
|
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20
Octinoxate LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-024 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.068 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-024-25 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-024-26 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-04-03 |
|
|
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20
Octinoxate LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-030 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.068 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-030-27 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-030-28 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-04-03 |
|
|
ESIKA HD COLOR HIGH DEFINITION COLOR SPF 20
Octinoxate LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-029 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.068 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-029-27 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-029-28 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-04-03 |
|
|














