EtoGesic
Boehringer Ingelheim Vetmedica, Inc.
EtoGesic® (etodolac) Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- Caution
- Description
- Indications
- Dosage and Administration
- Contraindications
- Warnings
- Precautions
- Side Effects
- Information for Dog Owners
- Clinical Pharmacology
- Effectiveness
- Animal Safety
- Storage
- How Supplied
- References
- 150 mg 30 count label
- 300 mg 30 count label
FULL PRESCRIBING INFORMATION
NADA 141-108, Approved by FDA
Non-steroidal anti-inflammatory for oral use in dogs only
Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
Etodolac is a pyranocarboxylic acid, chemically designated as (±) 1,8-diethyl-1,3,4,9-tetrahydropyrano-[3,4-b] indole-1-acetic acid. The structural formula for etodolac is shown:
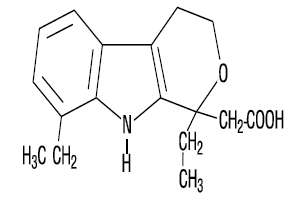
The empirical formula for etodolac is C17H21NO3. The molecular weight of the base is 287.37. It has a pKa of 4.65 and an n-octanol:water partition coefficient of 11.4 at pH 7.4. Etodolac is a white crystalline compound, insoluble in water but soluble in alcohols, chloroform, dimethyl sulfoxide, and aqueous poly-ethylene glycol. Each tablet is biconvex and half-scored and contains either 150 or 300 mg of etodolac.
Indications
EtoGesic Tablets are indicated for the control of pain and inflammation associated with osteoarthritis in dogs.
Dosage and Administration
Always provide Client Information sheet with prescription. Carefully consider the potential benefits and risks of EtoGesic and other treatment options before deciding to use EtoGesic. Use the lowest effective dose for the shortest duration consistent with individual treatment response.
The recommended dose of EtoGesic Tablets is 4.5 to 6.8 mg/lb body weight (10 to 15 mg/kg) administered once daily. Due to tablet sizes and scoring, dogs weighing less than 11 lb (5 kg) cannot be accurately dosed. The effective dose and duration should be based on clinical judgment of disease condition and patient tolerance of drug treatment. The initial dose level should be adjusted until a satisfactory clinical response is obtained, but should not exceed 15 mg/kg once daily.
Contraindications
EtoGesic Tablets are contraindicated in animals previously found to be hypersensitive to etodolac.
Warnings
Not for human use. Keep out of reach of children. Consult a physician in cases of accidental ingestion by humans. Do not use in cats. For use in dogs only.
All dogs should undergo a thorough history and physical examination before initiation of NSAID therapy. Appropriate laboratory tests to establish hematological and serum biochemical baseline data prior to, and periodically during, administration of any NSAID is recommended. Owners should be advised to observe for signs of potential drug toxicity (see Information for Dog Owners, Animal Safety, and Adverse Reactions) and be given a client information sheet about EtoGesic.
Precautions
The safe use of EtoGesic Tablets in dogs less than 12 months of age, pregnant, breeding or lactating dogs has not been established. Owners should be advised to observe for signs of potential drug reactions. If additional pain medication is warranted after administration of the daily dose of EtoGesic, alternative analgesia should be considered. The use of another NSAID is not recommended.
As a class, cyclooxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal, and hepatic toxicity. Sensitivity to drug-associated adverse effects varies with the individual patient. Dogs that have experienced adverse reactions from one NSAID may experience adverse reactions from other NSAIDs. Dogs at greatest risk for adverse events are those that are dehydrated, on concomitant diuretic therapy, or those with renal, cardiovascular, and/or hepatic dysfunction. Concurrent administration of potentially nephrotoxic drugs should be carefully approached and monitored. NSAIDs may inhibit the prostaglandins that maintain normal homeostatic function. Such anti-prostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that has not been previously diagnosed. Since NSAIDs possess the potential to induce gastrointestinal ulceration and/or gastrointestinal perforation, concomitant use of EtoGesic with other anti-inflammatory drugs, such as other NSAIDs or corticosteroids, should be avoided.
The use of concomitantly protein-bound drugs with EtoGesic has not been studied in dogs. Commonly used protein-bound drugs include cardiac, anticonvulsant and behavioral medications. The influence of concomitant drugs that may inhibit metabolism of EtoGesic has not been evaluated. Drug compatibility should be monitored in patients requiring adjunctive therapy. Consider appropriate washout times when switching from one NSAID to another or when switching from corticosteroid use to NSAID use.
Treatment with EtoGesic Tablets should be terminated if signs such as inappetence, emesis, fecal abnormalities, or anemia are observed. Dogs treated with non-steroidal anti-inflammatory drugs on a continuing basis, including etodolac, should be evaluated periodically to ensure that the drug is still necessary and well tolerated.
EtoGesic Tablets, as with other non-steroidal anti-inflammatory drugs, may exacerbate clinical signs in dogs with pre-existing or occult gastrointestinal, hepatic or cardiovascular abnormalities, blood dyscrasias, or bleeding disorders.
Side Effects
In a placebo-controlled field study with EtoGesic Tablets involving 116 dogs, where treatment was administered for 8 days, the following adverse reactions were noted:
|
Adverse Reaction |
EtoGesic Tablets % of dogs |
Placebo % of dogs |
| vomiting | 4.3 | 1.7 |
| regurgitation | 0.9 | 2.6 |
| lethargy | 3.4 | 2.6 |
| diarrhea/loose stool | 2.6 | 1.7 |
| hypoproteinemia | 2.6 | 0 |
| urticaria | 0.9 | 0 |
| behavioral change, urinating in house | 0.9 | 0 |
| inappetence | 0.9 | 1.7 |
Following completion of the field study, 92 dogs continued to receive etodolac tablets. One dog developed diarrhea following 2-1/2 weeks of treatment. Etodolac was discontinued until resolution of clinical signs was observed. When treatment was resumed, the diarrhea returned within 24 hours. One dog experienced vomiting which was attributed to treatment, and etodolac was discontinued.
Hypoproteinemia was identified in one dog following 11 months of etodolac therapy. Treatment was discontinued, and serum protein levels subsequently returned to normal.
EtoGesic Tablets Post-Approval Experience:
As with other drugs in the NSAID class, adverse responses to EtoGesic Tablets may occur. The adverse drug reactions listed below are based on voluntary post-approval reporting for EtoGesic Tablets. The categories of adverse reaction reports are listed below in decreasing order of frequency by body system.
Gastrointestinal: Vomiting, diarrhea, inappetence, gastroenteritis, gastrointestinal bleeding, melena, gastrointestinal ulceration, hypoproteinemia, elevated pancreatic enzymes.
Hepatic: Abnormal liver function test(s), elevated hepatic enzymes, icterus, acute hepatitis.
Hematological: Anemia, hemolytic anemia, thrombocytopenia, prolonged bleeding time.
Neurological/Behavioral/Special Senses: Ataxia, paresis, aggression, sedation, hyperactivity, disorientation, hyperesthesia, seizures, vestibular signs, keratoconjunctivitis sicca.
Renal: Polydipsia, polyuria, urinary incontinence, azotemia, acute renal failure, proteinuria, hematuria.
Dermatological/Immunological: Pruritus, dermatitis, edema, alopecia, urticaria.
Cardiovascular/Respiratory: Tachycardia, dyspnea.
In rare situations, death has been reported as an outcome of some of the adverse reactions listed above.
For technical assistance, to report a suspected adverse reaction, or obtain a Material Safety Data Sheet, call 1-866-638-2226.
Information for Dog Owners
EtoGesic, like other drugs of its class, is not free from adverse reactions. Owners should be advised of the potential for adverse reactions and be informed of the clinical signs associated with drug intolerance. Adverse reactions may include decreased appetite, vomiting, diarrhea, dark or tarry stools, increased water consumption, increased urination, pale gums due to anemia, yellowing of gums, skin or white of the eye due to jaundice, lethargy, incoordination, seizure, or behavioral changes. Serious adverse reactions associated with this drug class can occur without warning and in rare situations result in death (see Adverse Reactions). Owners should be advised to discontinue EtoGesic therapy and contact their veterinarian immediately if signs of intolerance are observed. The vast majority of patients with drug related adverse reactions have recovered when the signs are recognized, the drug is withdrawn, and veterinary care, if appropriate, is initiated. Owners should be advised of the importance of periodic follow-up for all dogs receiving a continuing regimen of any NSAID.
Clinical Pharmacology
Etodolac is a non-narcotic, non-steroidal anti-inflammatory drug (NSAID) with anti-inflammatory, anti-pyretic, and analgesic activity(1). The mechanism of action of etodolac, like that of other NSAIDs, is believed to be associated with inhibition of cyclooxygenase activity.
There are two main cyclooxygenase enzymes, COX-1 and COX-2, and a newly discovered third enzyme, COX-3, which has yet to be fully characterized(2). Cyclooxygenase-1 (COX-1) is the enzyme responsible for facilitating constitutive physiologic processes, e.g., platelet aggregation, gastric mucosal protection, and renal perfusion(3). It also is constitutively expressed in the brain, spinal cord, and reproductive tract(4). Cyclooxygenase-2 (COX-2) is responsible for the synthesis of inflammatory mediators, but it is also constitutively expressed in the brain, spinal cord and kidneys(5). COX-2 mRNA has been identified in the dog liver, ovary, lung, cerebral cortex and gastrointestinal tract(6). Cyclooxygenase-3 (COX-3) is constitutively expressed in the canine and human brain and the human heart(7).
In vitro experiments have shown that etodolac selectively inhibits COX-2 activity(8). Inhibition of COX-1 activity is associated with adverse effects on the gastrointestinal tract, whereas inhibition of COX-2 activity is associated with reducing inflammation. The clinical relevance of these data have not been shown. Etodolac also inhibits macrophage chemotaxis in vivo and in vitro (9). Because of the importance of macrophages in the inflammatory response, the anti-inflammatory effect of etodolac could be partially mediated through inhibition of the chemotactic ability of macrophages.
Pharmacokinetics in healthy beagle dogs: Etodolac is rapidly and almost completely absorbed from the gastrointestinal tract following oral administration. The extent of etodolac absorption (AUC) is not affected by the prandial status of the animal. However, it appears that the peak concentration of the drug decreases in the presence of food. As compared to an oral solution, the relative bioavailability of the tablets when given with or without food was essentially 100%. Peak plasma concentrations are usually attained within 2 hours of administration. Though the terminal half-life increases in a nonfasted state, minimal drug accumulation (less than 30%) is expected after repeated dosing (i.e., steady-state). Pharmacokinetic parameters estimated in a crossover study (fed vs. fasted) in eighteen 5-month old Beagle dogs are summarized in the following table:
| Pharmacokinetic Parameter | Tablet/Fasted | Tablet/Nonfasted |
| Cmax (mcg/mL) | 22.0 ± 6.42 | 16.9 ± 8.84 |
| Tmax (hr) | 1.69 ± 0.69 | 1.08 ± 0.46 |
| AUC0–∞(mcg·hours/mL) | 64.1 ± 17.9 | 63.9 ± 28.9 |
| Terminal half-life, T½(hrs) | 7.66 ± 2.05 | 11.98 ± 5.52 |
Pharmacokinetics of oral etodolac in dogs with reduced kidney function: In a study involving four Beagle dogs with induced acute renal failure, there was no observed change in drug bioavailability after administration of 200 mg single oral etodolac doses. In a study evaluating an additional four Beagles, no changes in electrolyte, serum albumin/total protein and creatinine concentrations were observed after single 200 mg doses of etodolac. This was not unexpected since the kidneys in normal dogs clear very little etodolac. Most of etodolac and its metabolites are eliminated via the liver and feces. In addition, etodolac is believed to undergo enterohepatic recirculation(10).
Effectiveness
A placebo-controlled, double-blinded field study demonstrated the anti-inflammatory and analgesic effectiveness of EtoGesic (etodolac) Tablets in various breeds of dogs. In this field study, dogs diagnosed with osteoarthritis secondary to hip dysplasia showed objective improvement in mobility as measured by force plate parameters when given EtoGesic Tablets at the label dosage for 8 days.
Animal Safety
In target animal safety studies, EtoGesic Tablets were well tolerated clinically when given at the label dosage for periods as long as one year (see Precautions).
Oral administration of etodolac at a daily dosage of 4.5 mg/lb (10 mg/kg) for twelve months or 6.8 mg/lb (15 mg/kg) for six months, resulted in some dogs showing a mild weight loss, fecal abnormalities (loose, mucoid, mucosanguineous feces or diarrhea), and hypoproteinemia. Erosions in the small intestine were observed in one of the eight dogs receiving 15 mg/kg following six months of daily dosing. In a separate pharmacokinetic study dogs were given the recommended dose level of EtoGesic Tablets daily for 28 consecutive days. This repeated treatment resulted in minimal drug accumulation.
Elevated dose levels of EtoGesic Tablets, i.e., ≥40 mg/kg/day (18 mg/lb/day, 2.7X the maximum daily dose), caused gastrointestinal ulceration, emesis, fecal occult blood, and weight loss. At a dose of ≥80 mg/kg/day (36 mg/lb/day, 5.3X the maximum daily dose), 6 of 8 treated dogs died or became moribund as a result of gastrointestinal ulceration. One dog died within 3 weeks of treatment initiation while the other 5 died after 3-9 months of daily treatment. Deaths were preceded by clinical signs of emesis, fecal abnormalities, decreased food intake, weight loss, and pale mucous membranes.
Renal tubular nephrosis was also found in 1 dog treated with 80 mg/kg for 12 months. Other common abnormalities observed at elevated doses included reductions in red blood cell count, hematocrit, hemoglobin, total protein and albumin concentrations; and increases in fibrinogen concentration and reticulocyte, leukocyte, and platelet counts.
In an additional study which evaluated the effects of EtoGesic Tablets administered to 6 dogs at the labeled dose for approximately 9.5 weeks, the incidence of stool abnormalities (diarrhea, loose stools) was unchanged for dogs in the weeks prior to initiation of treatment with EtoGesic Tablets, and during the course of this oral etodolac therapy. Five of the dogs receiving EtoGesic Tablets, versus 2 of the placebo-treated dogs, exhibited excessive bleeding during an experimental surgery. No significant evidence of drug-related toxicity was noted on necropsy.
Storage
Store at or below 25°C (77°F); brief excursions up to 30°C (86°F) are permitted.
How Supplied
EtoGesic (etodolac) Tablets are available in 150 and 300 mg single-scored tablets and supplied in bottles containing 30 and 90 tablets.
NDC 0010-3825-01 — 150 mg – bottles of 30
NDC 0010-3825-02 — 150 mg – bottles of 90
NDC 0010-3826-01 — 300 mg – bottles of 30
NDC 0010-3826-02 — 300 mg – bottles of 90
References
1. Kichiro, I, F Fujisawa, A Motonaga, Y Inoue, T Kyoi, F Ueda, K Kimura. Anti-inflammatory effects of etodolac: Comparison with other non-steroidal anti-inflammatory drugs. Biol. Pharm. Bull. (1994) 17:1577-1583
2. Willoughby DA, Moore AR and Colville-Nash PR. COX-1, COX-2, and COX-3 and the future treatment of chronic inflammatory disease. Lancet 2000;355:646-648.
3. Smith, et al., Pharmacological Analysis of Cyclooxygenase-1 in Inflammation. Proc. Natl. Acad. Sci. USA, Pharmacology 1998;95:13313-13318.
4. Jones CJ and Budsberg SC. Physiologic characteristics and clinical importance of the cyclooxygenase isoforms in dogs and cats. JAVMA 2000;217(5):721-729.
5. Zhang, et al., Inhibition of Cyco-oxygenase-2 Rapidly Reverses Inflammatory Hyperalgesia and Prostaglandin E2 Production. JPET 1997;283:1069-1075.
6. Wilson JE, et al. Determination of expression of cyclooxygenase -1 and -2 isozymes in canine tissues and their differential sensitivity to Nonsteroidal anti-inflammatory drugs. Am J Vet Res 2004; 65:810-818.
7. Chandrasekharan NV, Dai H, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure and expression. Proceedings of the National Academy of Sciences 2002;99(21):13926-13931.
8. Glaser, KB. Cyclooxygenase selectivity and NSAIDs: cyclooxygenase-2 selectivity of etodolac (Lodine®). Inflammopharmacol (1995) 3:335-345.
9. Gervais, F, RR Martel, E Skamene. The effect of the non-steroidal anti-inflammatory drug etodolac on macrophage migration in vitro and in vivo. J. Immunopharmacol (1984) 6:205-214.
10. Cayen, MN, M Kraml, ES Ferdinandi, EL Greselin, D Dvornik. The metabolic disposition of etodolac in rats, dogs, and man. Drug Metab. Revs. (1981) 12:339-362.
© 2010 Boehringer Ingelheim Vetmedica, Inc. All Rights Reserved.
EtoGesic is a registered trademark of Boehringer Ingelheim Vetmedica, Inc.
Manufactured by:
Boehringer Ingelheim Vetmedica, Inc.
St. Joseph, MO 64506 U.S.A.
12440 D5530A
EtoGesic® (pronounced et-toe-gee´-zik) for Osteoarthritis Pain
Generic name: etodolac (ee-toe-doe´-lak)
This summary contains important information about EtoGesic. You should read this information before you start giving your dog EtoGesic and should keep this sheet for future reference. This sheet is provided only as a summary and does not take the place of instructions from your veterinarian. Talk to your veterinarian if you do not understand any of this information or if you want to know more about EtoGesic.
What is EtoGesic?
EtoGesic is a nonsteroidal anti-inflammatory drug (NSAID) that is used to reduce pain and inflammation (soreness) due to osteoarthritis in dogs. Only a licensed veterinarian can prescribe EtoGesic for your dog. It is available as a tablet and is given to dogs by mouth.
Osteoarthritis (OA) is a painful condition caused by “wear and tear” of cartilage and other parts of the joints that may result in the following changes or signs in your dog:
• Limping or lameness
• Decreased activity or exercise (reluctance to stand, climb stairs, jump or run, or difficulty in performing these activities)
• Stiffness or decreased movement of joints.
What kind of results can I expect when my dog is on EtoGesic for OA?
While EtoGesic is not a cure for osteoarthritis, it can relieve the pain and inflammation of OA and improve your dog’s mobility.
• Response varies from dog to dog but can be quite dramatic.
• In most dogs, improvement can be seen in a matter of days.
• If EtoGesic is discontinued or not given as directed, your dog’s pain and inflammation may come back.
Who should not take EtoGesic?
Your dog should not be given EtoGesic if he/she:
• Has had an allergic reaction to etodolac, the active ingredient of EtoGesic.
• Has had an allergic reaction to aspirin or other NSAIDs (for example carprofen or phenylbutazone) such as hives, facial swelling, or red or itchy skin.
EtoGesic should be given to dogs only. Cats should not be given EtoGesic. Call your veterinarian immediately if your cat receives EtoGesic. People should not take EtoGesic. Keep EtoGesic and all medicine out of reach of children. Call your physician immediately if you accidentally take EtoGesic.
How to give EtoGesic to your dog.
EtoGesic should be given according to your veterinarian’s instructions. Your veterinarian will tell you what amount of EtoGesic is right for your dog and for how long it should be given. EtoGesic should be given by mouth and may be given with or without food.
What to tell/ask your veterinarian before giving EtoGesic.
Talk to your veterinarian about:
• The signs of OA you have observed (for example limping, stiffness).
• The importance of weight control and exercise in the management of OA.
• What tests might be done before EtoGesic is prescribed.
• How often your dog may need to be examined by your veterinarian.
• The risks and benefits of using EtoGesic.
Tell your veterinarian if your dog has ever had the following medical problems:
• Experienced side effects fromEtoGesic or other NSAIDs, such as aspirin
• Digestive upset (vomiting and/or diarrhea)
• Liver disease
• Kidney disease
• A bleeding disorder (for example, Von Willebrand’s disease)
Tell your veterinarian about:
• Any other medical problems or allergies that your dog has now or has had.
• All medicines that you are giving your dog or plan to give your dog, including those you can get without a prescription.
Tell your veterinarian if your dog is:
• Pregnant, nursing or if you plan to breed your dog.
What are the possible side effects that may occur in my dog during EtoGesic therapy?
EtoGesic, like other drugs, may cause some side effects. Serious but rare side effects have been reported in dogs taking NSAIDs, including EtoGesic. Serious side effects can occur with or without warning and in rare situations result in death.
The most common NSAID-related side effects generally involve the stomach (such as bleeding ulcers), and liver or kidney problems. Look for the following side effects that can indicate your dog may be having a problem with EtoGesic or may have another medical problem:
• Decrease or increase in appetite
• Vomiting
• Change in bowel movements (such as diarrhea, or black, tarry or bloody stools)
• Change in behavior (such as decreased or increased activity level, incoordination, seizure or aggression)
• Yellowing of gums, skin, or whites of the eyes (jaundice)
• Change in drinking habits (frequency, amount consumed)
• Change in urination habits (frequency, color, or smell)
• Change in skin (redness, scabs, or scratching)
It is important to stop therapy and contact your veterinarian immediately if you think your dog has a medical problem or side effect from EtoGesic therapy. If you have additional questions about possible side effects, talk to your veterinarian.
Can EtoGesic be given with other medicines?
EtoGesic should not be given with other NSAIDs (for example, aspirin, carprofen) or steroids (for example, cortisone, prednisone, dexamethasone, triamcinolone).
Tell your veterinarian about all medicines you have given your dog in the past, and any medicines that you are planning to give with EtoGesic. This should include other medicines that you can get without a prescription. Your veterinarian may want to check that all of your dog’s medicines can be given together.
What can I do in case my dog eats more than the prescribed amount?
Contact your veterinarian immediately if your dog eats more than the prescribed amount of EtoGesic.
What else should I know about EtoGesic?
This sheet provides a summary of information about EtoGesic. If you have any questions or concerns about EtoGesic or osteoarthritis pain, talk to your veterinarian.
As with all prescribed medicines, EtoGesic should only be given to the dog for which it was prescribed. It should be given to your dog only for the condition for which it was prescribed.
It is important to periodically discuss your dog’s response to EtoGesic at regular check ups. Your veterinarian will best determine if your dog is responding as expected and if your dog should continue receiving EtoGesic.
How do I report a suspected adverse reaction?
To report a suspected adverse reaction, please call 1-866-638-2226.
EtoGesic is a registered trademark of Boehringer Ingelheim Vetmedica, Inc.
©2010 Boehringer Ingelheim Vetmedica, Inc. All Rights Reserved.
Manufactured by:
Boehringer Ingelheim Vetmedica, Inc.
St. Joseph, MO 64506 U.S.A.
12440 D5530A
150 mg 30 count label

300 mg 30 count label

EtoGesicetodolac TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EtoGesicetodolac TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||