Excede
Pharmacia & Upjohn Company
Pfizer Inc.
EXCEDE (Ceftiofur Crystalline Free Acid) Sterile Suspension
FULL PRESCRIBING INFORMATION: CONTENTS*
- CAUTION
- EXCEDE DESCRIPTION
- INDICATIONS
- DOSAGE
- ADMINISTRATION
- ADMINISTRATION FOR BASE OF THE EAR
- EXCEDE CONTRAINDICATIONS
- WARNINGS
- RESIDUE WARNINGS
- ANTIBACTERIAL WARNINGS
- PRECAUTIONS
- ADVERSE EFFECTS
- CLINICAL PHARMACOLOGY
- EFFECTIVENESS
- ANIMAL SAFETY
- TISSUE AND MILK RESIDUE DEPLETION
- STORAGE CONDITIONS
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 200 mg Bottle Label
- PRINCIPAL DISPLAY PANEL - 200 mg Bottle Carton
FULL PRESCRIBING INFORMATION
For subcutaneous injection in the posterior aspect of the ear where it attaches to the head (base of the ear) in lactating dairy cattle. For subcutaneous injection in the middle third of the posterior aspect of the ear or in the posterior aspect of the ear where it attaches to the head (base of the ear) in beef and non-lactating dairy cattle. Not for use in calves to be processed for veal.
CAUTION
Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
EXCEDE DESCRIPTION
EXCEDE Sterile Suspension is a ready-to-use formulation that contains the crystalline free acid of ceftiofur, which is a broad spectrum cephalosporin antibiotic active against Gram-positive and Gram-negative bacteria including β-lactamase-producing strains. Like other cephalosporins, ceftiofur is bactericidal, in vitro, resulting from inhibition of cell wall synthesis.
Each mL of this ready-to-use sterile suspension contains ceftiofur crystalline free acid equivalent to 200 mg ceftiofur, in a caprylic/capric triglyceride (Miglyol®) and cottonseed oil based suspension.
Figure 1. Structure of ceftiofur crystalline free acid:

Chemical name of ceftiofur crystalline free acid:
7-[[2-(2-Amino-4-thiazolyl)-2-(methoxyimino)acetyl]amino]- 3-[[(2-furanylcarbonyl) thio] methyl]-8-oxo-5-thia-1- azabicyclo[4.2.0]oct-2-ene 2-carboxylic acid
INDICATIONS
EXCEDE Sterile Suspension is indicated for treatment of bovine respiratory disease (BRD, shipping fever, pneumonia) associated with Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni in beef, non-lactating dairy, and lactating dairy cattle.
EXCEDE Sterile Suspension is also indicated for the control of respiratory disease in beef and non-lactating dairy cattle which are at high risk of developing BRD associated with M. haemolytica, P. multocida, and H. somni.
EXCEDE Sterile Suspension is also indicated for the treatment of bovine foot rot (interdigital necrobacillosis) associated with Fusobacterium necrophorum and Porphyromonas levii in beef, non-lactating dairy, and lactating dairy cattle.
EXCEDE Sterile Suspension is also indicated for treatment of acute metritis (0-10 days post-partum) associated with bacterial organisms susceptible to ceftiofur in lactating dairy cattle.
DOSAGE
Treatment of BRD and bovine foot rot
Administer as a single subcutaneous injection in the posterior aspect of the ear where it attaches to the head (base of the ear) to cattle at a dosage of 3.0 mg ceftiofur equivalents (CE)/lb (6.6 mg CE/kg) body weight (BW) (1.5 mL sterile suspension per 100 lb BW).
In beef and non-lactating dairy cattle, EXCEDE Sterile Suspension may also be administered as a single subcutaneous injection in the middle third of the posterior aspect of the ear at a dosage of 3.0 mg CE/lb (6.6 mg CE/kg) BW (1.5 mL sterile suspension per 100 lb BW).
Most animals will respond to treatment within three to five days. If no improvement is observed, the diagnosis should be reevaluated.
Control of BRD
Administer as a subcutaneous injection either in the middle third of the posterior aspect of the ear or in the posterior aspect of the ear where it attaches to the head (base of the ear) to beef and non-lactating dairy cattle at a dosage of 3.0 mg CE/lb (6.6 mg CE/kg) BW (1.5 mL sterile suspension per 100 lb BW).
Clinical studies indicate that administration of EXCEDE Sterile Suspension is effective for the control of respiratory disease in beef and non-lactating dairy cattle at "high risk" of developing BRD. One or more of the following factors typically characterizes calves on arrival at high risk of developing BRD.
- Cattle are from multiple farm origins,
- cattle have had extended transport times (that may have included few if any rest stops),
- ambient temperature change from origin to arrival of 30° F or more,
- cattle have had continued exposure to extremely wet or cold weather conditions,
- cattle have experienced excessive shrink or excessive arrival processing procedures (such as castration, dehorning).
Treatment of Acute Metritis
Administer as a subcutaneous injection in the posterior aspect of the ear where it attaches to the head (base of the ear) to lactating dairy cattle at a dosage of 3.0 mg CE/lb (6.6 mg CE/kg) BW (1.5 mL sterile suspension per 100 lb BW). Repeat this dose in the contra-lateral (opposite) ear approximately 72 hours following the initial dose.
|
Weight (lb) |
Dose Volume (mL) |
Weight (lb) |
Dose Volume (mL) |
|
| 100 | 1.5 | 1100 | 16.5 | |
| 200 | 3.0 | 1200 | 18.0 | |
| 300 | 4.5 | 1300 | 19.5 | |
| 400 | 6.0 | 1400 | 21.0 | |
| 500 | 7.5 | 1500 | 22.5 | |
| 600 | 9.0 | 1600 | 24.0 | |
| 700 | 10.5 | 1700 | 25.5 | |
| 800 | 12.0 | 1800 | 27.0 | |
| 900 | 13.5 | 1900 | 28.5 | |
| 1000 | 15.0 | 2000 | 30.0 |
ADMINISTRATION
ADMINISTRATION FOR THE MIDDLE THIRD OF THE EAR
- Shake well before using. Please read the complete package insert before administering EXCEDE Sterile Suspension subcutaneously in the posterior ear of cattle.
- Deposit as a single subcutaneous injection in the middle third of the posterior aspect of the ear, avoiding all blood vessels. See Figures 2 and 3.
- Adjust the needle insertion point to avoid any blood vessels, previous implants, ear tags or ear tag holes. Do not administer intra-arterially.
- Deliver the entire contents of the syringe.
- When administered correctly, a subcutaneous bleb of EXCEDE Sterile Suspension will appear.
- When withdrawing the needle, apply pressure to the needle insertion point, and massage toward the base of the ear.
Figure 2. Subcutaneous administration of EXCEDE Sterile Suspension in the middle third of the posterior aspect of the ear.

Figure 3. Diagram of the approximate locations of the major arteries of the posterior ear and the recommended needle insertion locations. Administration of EXCEDE Sterile Suspension into ear arteries is likely to be fatal.

ADMINISTRATION FOR BASE OF THE EAR
In lactating dairy cattle the injection techniques for subcutaneous (SC) injection in the posterior aspect of the ear where it attaches to the head (base of the ear) can be made by the rostral or ventral injection techniques.
In beef and non-lactating dairy cattle the SC injection in the base of the ear can be made by the rostral, ventral or toward the opposite eye injection techniques.
- Shake well before using. Please read the complete package insert before administering EXCEDE Sterile Suspension subcutaneously in the posterior aspect of the ear where it attaches to the head (base of the ear).
- The subcutaneous (SC) injection may be made using the toward the opposite eye, rostral, or ventral techniques. Hold the syringe and needle and insert the needle as described below.
- Deliver the entire contents of the syringe.
- Do not administer EXCEDE Sterile Suspension in the neck.
Administration for the Base of the Ear: Toward the Opposite Eye Technique
- Hold the syringe and needle behind the ear to be dosed so the needle and syringe point in the direction of an imaginary line that would pass through the head toward the animal's opposite eye. See Figures 4 and 5.
- Insert the needle through the loose skin in the posterior aspect of the ear where it attaches to the head (base of the ear) while maintaining this angle. See Figure 4.
Figure 4. Subcutaneous administration of EXCEDE Sterile Suspension in the posterior aspect of the ear where it attaches to the head (base of the ear).

Figure 5. Injection location for the subcutaneous administration of EXCEDE Sterile Suspension in the posterior aspect of the ear where it attaches to the head (base of the ear).

Administration for the Base of Ear: Toward the Same Eye Technique or Rostral Direction
- Hold the syringe and needle behind the ear to be dosed so the needle and syringe point in the direction of an imaginary line that would pass through the head toward the eye on the same side of the head. See Figures 5 and 6.
- Insert the needle through the loose skin in the posterior aspect of the ear where it attaches to the head (base of the ear) while maintaining the needle position. See Figure 6.
Figure 6. Diagram of head showing the direction for the base of ear injections administered rostrally toward the eye on the same side of the head into the loose skin in the caudal aspect of the base of the ear.
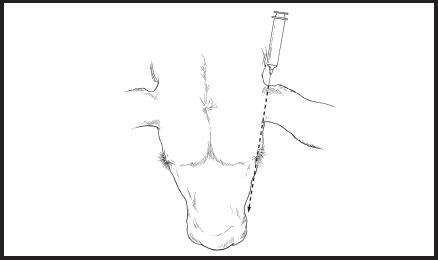
Administration for Base of the Ear: Ventral Technique
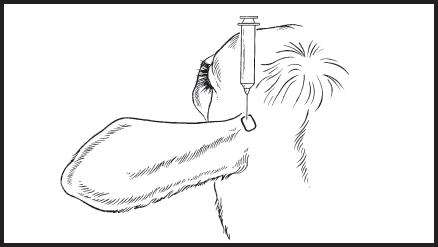
- Hold the syringe and needle above the ear to be dosed so that the needle and syringe are pointing ventrally toward the base of the ear. The needle will be inserted into the loose skin in the posterior aspect of the ear where it attaches to the head (base of the ear) while pointing ventrally. Care should be taken to not insert the needle through the cartilage of the ear. See Figure 7.
- Insert the needle through the loose skin in the posterior aspect of the ear where it attaches to the head (base of the ear) while maintaining needle position. See Figure 7.
Figure 7. Diagram of head showing the direction of base of ear injections when administered ventrally into the loose skin in the caudal aspect of the base of the ear.
EXCEDE CONTRAINDICATIONS
As with all drugs, the use of EXCEDE Sterile Suspension is contraindicated in animals previously found to be hypersensitive to the drug.
WARNINGS
FOR USE IN ANIMALS ONLY. NOT FOR HUMAN USE.
KEEP OUT OF REACH OF CHILDREN.
Penicillins and cephalosporins can cause allergic reactions in sensitized individuals. Topical exposures to such antimicrobials, including ceftiofur, may elicit mild to severe allergic reactions in some individuals. Repeated or prolonged exposure may lead to sensitization. Avoid direct contact of the product with the skin, eyes, mouth and clothing. Sensitization of the skin may be avoided by wearing protective gloves.
Persons with a known hypersensitivity to penicillin or cephalosporins should avoid exposure to this product.
In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. If allergic reaction occurs (e.g., skin rash, hives, difficult breathing), seek medical attention.
The material safety data sheet contains more detailed occupational safety information. To obtain a material safety data sheet please call 1-800-733-5500. To report any adverse event please call 1-800-366-5288.
Intra-arterial injection may occur during administration of EXCEDE Sterile Suspension via middle third of the ear injection or base of the ear injection directed towards the opposite eye. Intra-arterial injection of EXCEDE Sterile Suspension is likely to result in sudden death of the animal.
RESIDUE WARNINGS
- Following label use as either a single-dose or 2-dose regimen, a 13-day pre-slaughter withdrawal period is required after the last treatment.
- Following label use as either a single-dose or 2-dose regimen, no milk discard period is required for this product.
- Use of dosages in excess of 3.0 mg CE/lb (6.6 mg CE/kg) BW or administration by unapproved routes (subcutaneous injection in the neck or intramuscular injection) may cause violative residues.
- A withdrawal period has not been established for this product in preruminating calves.
- Do not use in calves to be processed for veal.
ANTIBACTERIAL WARNINGS
Use of antibacterial drugs in the absence of a susceptible bacterial infection is unlikely to provide benefit to treated animals and may increase the risk of the development of drug-resistant bacteria.
PRECAUTIONS
Following subcutaneous injection in the middle third of the posterior aspect of the ear, thickening and swelling (characterized by aseptic cellular infiltrate) of the ear may occur. As with other parenteral injections, localized post-injection bacterial infections may result in abscess formation. Attention to hygienic procedures can minimize their occurrence.
Following injection in the posterior aspect of the ear where it attaches to the head (base of the ear), areas of discoloration and signs of inflammation may persist at least 13 days post administration resulting in trim loss of edible tissue at slaughter. Injection of volumes greater than 20 mL, in the middle third of the ear, may result in open draining lesions in a small percentage of cattle.
The effects of ceftiofur on bovine reproductive performance, pregnancy, and lactation have not been determined.
ADVERSE EFFECTS
Intra-arterial injection may occur during administration of EXCEDE Sterile Suspension via middle third of the ear injection or base of the ear injection directed towards the opposite eye. Intra-arterial injection of EXCEDE Sterile Suspension is likely to result in sudden death of the animal. During the conduct of clinical studies, there was a low incidence of acute death (see ANIMAL SAFETY) confirmed to be the result of inadvertent intra-arterial injection. No other adverse systemic effects were noted for either the antibiotic or formulation during any of the clinical and target animal safety studies.
CLINICAL PHARMACOLOGY
Ceftiofur administered as either ceftiofur sodium (NAXCEL® Sterile Powder), ceftiofur hydrochloride (EXCENEL® RTU Sterile Suspension), or ceftiofur crystalline free acid (EXCEDE Sterile Suspension) is metabolized rapidly to desfuroylceftiofur, the primary metabolite. Subcutaneous administration of ceftiofur crystalline free acid, either in the middle third of the posterior aspect of the ear (middle third of the ear, MOE) of beef and non-lactating dairy cattle, or in the posterior aspect of the ear where it attaches to the head (base of the ear, BOE) of beef, non-lactating dairy, and lactating dairy cattle, provides therapeutic concentrations of ceftiofur and desfuroylceftiofur-related metabolites in plasma above the lowest minimum inhibitory concentration to encompass 90% of the most susceptible isolates (MIC90) for the labeled BRD pathogens, Pasteurella multocida, Mannheimia haemolytica and Histophilus somni, for generally not less than 150 hours after a single administration (See Figure 8).
Single Dose Regimen
The pharmacokinetic parameters for the two subcutaneous locations of injection (MOE and BOE) are found in Table 2. Statistical analyses of the data from these two subcutaneous injection sites (MOE and BOE) demonstrate that they are therapeutically equivalent.
Figure 8. Average (n=12/group) plasma concentrations of ceftiofur and desfuroylceftiofur-related metabolites after administration of EXCEDE Sterile Suspension at 3.0 mg CE/lb (6.6 mg CE/kg) BW via subcutaneous injection into one of two different locations of the ear, middle third of the ear (MOE Cattle) and base of the ear (BOE Cattle) in beef cattle as well into the base of the ear (BOE Lactating) in lactating dairy cattle.

| Pharmacokinetic Parameter | Beef - Middle Third of the Ear Mean Value ± Standard Deviation | Beef - Base of the Ear Mean Value ± Standard Deviation | Dairy Cow - Base of the Ear Mean Value ± Standard Deviation |
|---|---|---|---|
| Cmax (µg CE/mL) = maximum plasma concentration (in µg CE/mL). tmax (h) = the time after injection when Cmax occurs (in hours). AUC 0-LOQ (µg∙h/mL) = the area under the plasma concentration vs. time curve from time of injection to the limit of quantitation of the assay (0.15 µg CE/mL). t>0.2, model (h) = the time plasma concentrations remain above 0.2 µg CE/mL (in hours), estimated using compartmental pharmacokinetic techniques. t>0.2, nca (h) = the time plasma concentrations remain above 0.2 µg CE/mL (in hours), estimated using noncompartmental pharmacokinetic techniques. t1/2 (h) = terminal phase biological half life (in hours) NE = Not estimated |
|||
| Cmax (µg CE/mL) | 6.90 ± 2.68 | 6.39 ± 1.79 | 4.44 ± 1.65 |
| tmax (h) | 12.0 ± 6.2 | 19.8 ± 5.81 | 19.00 ± 8.02 |
| AUC 0-LOQ (µg∙h/mL) | 376 ± 66.1 | 412 ± 67.3 | 313 ± 85.5 |
| t>0.2, model (h) | 183 ± 40.8 | NE | NE |
| t>0.2, nca (h) | 246 ± 48.5 | 218 ± 45.5 | 205 ± 35.7 |
| t1/2 (h) | 62.3 ± 13.5 | 40.7 ± 11.2 | 43.92 ± 9.84 |
Two-Dose Regimen
A two-dose regimen of 6.6 mg CE/kg BW administered 72 hours apart is required for the treatment of acute metritis in lactating cows. The mean plasma concentration vs. time profile for ceftiofur and desfuroylceftiofur-related metabolites for the 2-dose regimen in 12 cows is shown in Figure 9 below. The pharmacokinetic parameters for the 2-dose regimen are provided in Table 3.
Figure 9. LS-Mean DCA Plasma Concentration Time Profile Following Two Subcutaneous Injections of EXCEDE 72 hours apart at a Dose of 3.0 mg CE/lb (6.6 mg CE/kg) BW in 12 lactating cows.

| PK Parameter | Mean ± Standard Deviation |
|---|---|
| AUC0-LOQ (µg∙h/mL) | 651 ± 119 |
| t½ (h) | 55.7 ± 4.84 |
| t>0.2 (h) | 341 ± 34.0 |
| Tmax (h) | 77.1 ± 33.4 |
| Cmax (µg/mL) | 5.98 ± 2.51 |
MICROBIOLOGY
Ceftiofur has demonstrated in vitro activity against Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni, three major pathogens associated with BRD, and against Fusobacterium necrophorum and Porphyromonas levii associated with bovine foot rot.
A summary of the susceptibility of BRD and foot rot pathogens is presented in Table 4. BRD isolates were obtained from cattle enrolled in a field study conducted in the United States that were diagnosed with BRD. Foot rot isolates were obtained from cattle enrolled in a field study conducted in the United States and Canada that were diagnosed with foot rot. Susceptibility testing was conducted according to the Clinical and Laboratory Standards Institute (CLSI) M7-A3 and M11-A6 standards for BRD and foot rot isolates, respectively.
| Indicated pathogen | Year of isolation | Number of isolates | MIC50
 |
MIC90
 |
MIC range (µg/mL) |
|---|---|---|---|---|---|
| Mannheimia haemolytica | 1996 to 1997 | 75 | 0.008 | 0.015 | 0.001 to 0.015 |
| Pasteurella multocida | 1996 to 1997 | 43 | 0.004 | 0.004 | 0.001 to 0.015 |
| Histophilus somni | 1996 to 1997 | 11 | 0.004 | 0.004 | 0.002 to 0.015 |
| Fusobacterium necrophorum | 2006 to 2007 | 148 | ≤ 0.25 | 0.5 | ≤ 0.25 to >128 |
| Porphyromonas levii | 2006 to 2007 | 141 | ≤ 0.25 | 2.0 | ≤ 0.25 to 16 |
Based on pharmacokinetic and clinical effectiveness studies of ceftiofur in cattle after a single administration of 3.0 mg CE/lb (6.6 mg CE/kg) BW and the MIC and susceptibility data, the following breakpoints are recommended for BRD pathogens by CLSI.
| Pathogen | Disk potency | Zone diameter (mm) |
MIC breakpoint (µg/mL) |
||||
|---|---|---|---|---|---|---|---|
| S | I | R | S | I | R | ||
| S – Susceptible I – Intermediate R – Resistant |
|||||||
|
Mannheimia haemolytica Pasteurella multocida Histophilus somni |
30 µg | ≥ 21 | 18 to 20 | ≤ 17 | ≤ 2.0 | 4.0 | ≥ 8.0 |
EFFECTIVENESS
A field dose confirmation study for the treatment of BRD evaluated the effectiveness of single doses of 2.0 and 3.0 mg CE/lb (4.4 or 6.6 mg CE/kg) BW for the treatment of the bacterial component of BRD under field conditions. All treatments were administered subcutaneously in the middle third of the posterior aspect of the ear. Cattle were clinically evaluated on Days 2 to 4, 14 and 28 and were observed on all other study days. The 3.0 mg CE/lb (6.6 mg CE/kg) BW EXCEDE Sterile Suspension dose significantly (p ≤ 0.05) increased Day 14 treatment success rate, defined as animals that did not require any ancillary treatment and had a rectal temperature of < 104°F, normal respiration index, and had no or mild depression on that day.
The effectiveness of a single dose of EXCEDE Sterile Suspension for the control of BRD in feedlot cattle was evaluated in a nine-location field effectiveness study. In addition to standard processing on arrival at feedlots, cattle (n=3911) considered to be at high risk for BRD were assigned to one of four arrival treatments, including EXCEDE Sterile Suspension at 2.0 or 3.0 mg CE/lb (4.4 or 6.6 mg CE/kg) BW or negative control. Effectiveness evaluation was based on the incidence of clinical BRD within 28 days following arrival processing. Administration of a single dose of EXCEDE Sterile Suspension administered subcutaneously in the middle third of the posterior aspect of the ear at arrival processing significantly reduced the incidence of BRD in high-risk feedlot cattle in the 28-day period after arrival processing compared to negative controls.
Base of the ear administration (beef and non-lactating dairy cattle) and middle third of the ear administration (lactating dairy cattle) were compared to the middle third of the ear pharmacokinetic data for beef and non-lactating dairy cattle and were found to be therapeutically equivalent.
The effectiveness of EXCEDE Sterile Suspension for the treatment of bovine foot rot was evaluated in a six-location field effectiveness study. Cattle diagnosed with bovine foot rot were enrolled and treated with EXCEDE Sterile Suspension, administered by subcutaneous injection in the base of the ear as a single dose of 3.0 mg CE/lb (6.6 mg CE/kg) BW or an equivalent volume of a vehicle control. Cattle were clinically evaluated 7 days post-treatment for treatment success, which was based on defined decreases in lesion, swelling and lameness scores. A total of 169 beef and dairy cattle were included in the analysis. There was a statistically significant difference (p = 0.0054) in treatment success for EXCEDE-treated cattle (58.4%) compared to vehicle-treated control cattle (13.2%).
The effectiveness of EXCEDE Sterile Suspension for the treatment of acute metritis was evaluated in a 15-location field effectiveness study. A total of 1023 cows with a fetid vaginal discharge and a rectal temperature of ≥ 103 °F were enrolled in the study and treated with either a two-dose regimen of EXCEDE (6.6 mg CE/BW) or an equivalent volume of vehicle control, administered approximately 72 hours apart at the base of opposite ears. At 14 days post-treatment, each cow remaining in the study was examined and rectal temperature and vaginal discharge score were recorded. Cows with a non-fetid discharge, and a rectal temperature < 103 °F, and that did not require alternate ("escape") therapy during the 14_day observation period were classified as a cure. The cure rate was significantly higher (p < 0.0001) in EXCEDE-treated cows (362/493, 74.3%) than in vehicle-treated cows (271/489, 55.3%). One cow died 15 to 20 minutes after the second administration of EXCEDE. Necropsy findings determined the probable cause of death to be intra-arterial injection.
ANIMAL SAFETY
Systemic Safety Studies
After parenteral administration, ceftiofur crystalline free acid (as EXCEDE Sterile Suspension), ceftiofur sodium and ceftiofur hydrochloride are rapidly metabolized to desfuroylceftiofur. Therefore, studies conducted with ceftiofur sodium are adequate to evaluate the systemic safety of EXCEDE Sterile Suspension. Results from a five-day tolerance study conducted with ceftiofur sodium in normal feeder calves indicated that ceftiofur was well tolerated at 25 mg CE/lb/day for five consecutive days, approximately 8 times the approved dose of EXCEDE Sterile Suspension 3.0 mg CE/lb (6.6 mg CE/kg) BW. Ceftiofur administered parenterally had no adverse systemic effects.
In a 15-day safety/toxicity study, five steer and five heifer calves per group were administered ceftiofur sodium intramuscularly at 0 (vehicle control), 1, 3, 5 or 10 mg CE/lb/day thus, evaluating up to 3.3 times the approved dose of EXCEDE Sterile Suspension of 3.0 mg CE/lb/day (6.6 mg CE/kg) BW. There were no adverse systemic effects, indicating that ceftiofur has a wide margin of safety when injected intramuscularly into feeder calves. Local tissue tolerance to subcutaneous injection of EXCEDE Sterile Suspension in the posterior ear of cattle was evaluated in a separate study.
The systemic safety of ceftiofur concentrations resulting from product administration at the base of the ear was established via a pharmacokinetic comparison of the two routes of administration (base of the ear versus middle third of the ear). Based upon the results of this relative bioavailability study, it was determined that the two routes of administration are therapeutically equivalent.
To support systemic target animal safety for the 2-dose metritis regimen, five projected daily doses of NAXCEL Sterile Powder (ceftiofur sodium) at 2.2 mg/kg BW were compared pharmacokinetically with EXCEDE administered 2 times at a 72 hour interval at 6.6 mg/kg BW. The peak concentration (Cmax) and the extent of exposure (AUC) after two doses of EXCEDE were statistically no higher than the exposure following five daily doses of NAXCEL Sterile Powder in beef cattle.
Investigation of Intra-Arterial and Intravenous Injection
In approximately 6000 animals enrolled in the BRD clinical studies, nine animals died following injection of EXCEDE Sterile Suspension. All deaths were within 30 minutes of the time of injection. The exact cause was confirmed in three animals. These deaths resulted from inadvertent intra-arterial injection of this oil-based suspension into one of the two major auricular (ear) arteries. Intra-arterial injection at this location resulted in direct administration of the oil-based formulation into the arterial blood supply of the brain resulting in embolism and death.
Since intra-arterial injection was confirmed in three animals that died following injection of EXCEDE Sterile Suspension, the consequences of purposeful intra-arterial injection of EXCEDE Sterile Suspension were investigated in feeder cattle. Two heifers (body weight approximately 225 kg) were given a single 3.0 mg CE/lb (6.6 mg CE/kg) BW bolus dose of EXCEDE Sterile Suspension in the middle auricular artery. Both heifers collapsed immediately and died within approximately eight minutes of injection. Intra-arterial injection of EXCEDE Sterile Suspension in the ear will result in death and must be avoided.
Since subcutaneous injection in the ear may potentially result in inadvertent intravenous administration of an injectable product, the consequences of purposeful intravenous injection of EXCEDE Sterile Suspension were investigated in feeder cattle. Three heifers and three steers (body weight range 197-223 kg) were given a single 3.0 mg CE/lb (6.6 mg CE/kg) BW bolus dose of EXCEDE Sterile Suspension in the jugular vein and were monitored for adverse effects following injection. One steer and one heifer had transient (2 to 5 minutes) increases in heart rate without any other untoward signs in these or the other cattle. Intravenous injection of EXCEDE Sterile Suspension is an unacceptable route of administration.
Safety Studies in Beef Cattle
Middle of the ear injection
A study was designed and conducted to specifically address tissue tolerance in cattle when EXCEDE Sterile Suspension was administered as a single subcutaneous injection into the posterior aspect of the ear of cattle at the recommended dose of 3.0 mg CE/lb (6.6 mg CE/kg) BW. Results from this study indicate that the subcutaneous injection of EXCEDE Sterile Suspension into the middle third of the posterior aspect of the ear of cattle is well tolerated and characterized by a biphasic thickening of the ear. The initial increase in thickness is attributed to the space required for the volume of injected material. Additional increases in thickness were observed through Day 14 after injection. After Day 14, post injection ear thickness decreased in all animals. One animal carried an injected ear in a drooping position for 7 days post injection. At necropsy, subcutaneous areas of discoloration and some foci of hemorrhage were observed in ears of injected cattle. The discoloration was markedly reduced in size by the end of the study. Ears are inedible tissues in the US (9 CFR 301.2). No signs of irritation were observed on the edible portions of the carcass around the base of the ear.
The local tolerance of the ear of cattle to a single subcutaneous injection of EXCEDE Sterile Suspension was also evaluated in a large multi-location effectiveness study. None of the 1927 animals treated with EXCEDE Sterile Suspension were removed from this trial due to ear irritation although swelling was noted at some injection sites. Leak back and/or bleeding from the injection site was observed in a small fraction of the treated animals immediately after administration. It was concluded that administration of EXCEDE Sterile Suspension in the posterior aspect of the ear was well tolerated and was acceptable under feedlot conditions.
A study evaluated the 56-day feedlot performance of beef steers administered EXCEDE Sterile Suspension alone, EXCEDE Sterile Suspension with a growth promoting implant, growth promoting implant alone, or neither product, in a total of 207 Angus and Angus cross-bred steers. The administration of EXCEDE Sterile Suspension in the posterior aspect of the ear with or without growth promoting implants was well tolerated by cattle and did not adversely affect feedlot cattle performance. Based upon the results of this study, the location of implants administered after EXCEDE Sterile Suspension may need to be adjusted slightly within the boundaries of the middle third of the ear in some animals.
Base of the ear injection
The local tolerance of the ear to a single subcutaneous injection at the base of the ear of EXCEDE Sterile Suspension was evaluated in a multi-location field study in 2926 beef cattle. Normal restraint was adequate for administration of EXCEDE Sterile Suspension for 99.8% of cattle. No post injection problems (excessive bleeding or leak back) were observed in 99.8% of cattle. On Days 28 and 56 post-injection, 97.8% and 98.9% of the cattle had "normal" (no observed swelling) ears.
In a residue study, 72 beef cattle were injected in the base of the ear with EXCEDE Sterile Suspension at a dose rate of 3.0 mg CE/lb (6.6 mg CE/kg) BW. Injection sites were observed daily from treatment to necropsy (4, 7, 10, or 13 days post-injection) for swelling and drooping, and evaluated grossly at necropsy, using skinning and trimming procedures similar to slaughterhouse practices. All animals had injection site swelling during the study; swelling resolved prior to euthanasia in 23 of 72 animals. None of the animals showed ear drooping. At necropsy, signs of inflammation (hemorrhage, congestion, and firmness of tissue) and presence of drug material were seen in the area around the injection site and on the carcass. At 13 days post-injection, gross lesions were found in the inedible portions of the base of the ear in all 18 animals, and in the exposed carcass tissue in 11 of 18 animals.
The ventral base of the ear injection technique was evaluated in a conditions of use study in 200 beef cattle. Each animal received a single injection of EXCEDE Sterile Suspension at a dose of 6.6 mg CE/kg BW at the base of the ear using the ventral injection technique. Normal restraint was adequate for 95.5% of animals in the study. Injection site scores were normal for 65.3% and 92.5% of cattle on Days 14 and 28, respectively. One animal had an unusually large swelling on Day 7 which reduced to a size comparable to other study animals by Day 14.
Safety Studies in Lactating Dairy Cattle
The local tolerance of the ear to a single subcutaneous injection at the base of the ear of EXCEDE Sterile Suspension was evaluated in a multi-location field study in 114 adult dairy cattle. Successful injection in the base of the ear was achieved in 97.4% of cattle using normal facilities and restraint equipment. No leak back or excessive bleeding was observed following injection for 99.1% of cattle, with injection volumes ranging from 15 to 30 mL. On Days 28 and 56 following injection of EXCEDE Sterile Suspension in the base of the ear, 95.6% and 100% of ears, respectively, were observed as normal with no injection site swelling.
In a residue study, six dairy cows were injected in the base of the ear at a dose rate of 3.0 mg CE/lb (6.6 mg CE/kg) BW of EXCEDE Sterile Suspension. No animals exhibited drooping ears at any time after treatment but all animals had signs of swelling at the injection site at all observation times after treatment. Cows were slaughtered 10 days after injection. At necropsy, all six cows showed evidence of injection site inflammation (discoloration of fat tissue/fascia) and four of six cows had discoloration of tissue dorsal and posterior to the ear canal on the carcass. In addition to discoloration, tan nodules and a milky white fluid exudate were also present at the sectioned surface.
Injection site safety for base of the ear administration was evaluated in the metritis effectiveness study described above. Normal restraint was adequate for ≥ 97.8% of injections administered. Injection site scores were normal in 50.3%, 73.2%, and 96.4% at 2 or 3, 11, and 54±3 days after the second injection, respectively.
The ventral and rostral base of the ear injection techniques were compared with the toward the opposite eye technique in a conditions of use study in 197 lactating dairy cattle. Normal restraint was adequate for 89.8% (ventral), 98% (rostral), and 100% (opposite eye) of animals in the study. Injection site scores were normal for 32% (rostral), 46.9% (ventral), and 47.9% (opposite eye) of cattle on Day 14, and 73% (rostral), 87.8% (ventral), and 64.6% (opposite eye) of cattle on Day 28, respectively.
TISSUE AND MILK RESIDUE DEPLETION
A radiolabeled residue metabolism study established tolerances for ceftiofur residues in cattle kidney, liver and muscle. A separate study established the tolerance for ceftiofur residues in milk. The tolerances for ceftiofur residues are 0.4 ppm in kidney, 2.0 ppm in liver, 1.0 ppm in muscle and 0.1 ppm in milk.
A pivotal tissue residue decline study was conducted in dairy cattle. In this study, cows received a single injection of 3.0 mg CE/lb (6.6 mg CE/kg) BW. Ceftiofur residues in tissues were less than the tolerances for ceftiofur residues in tissues such as the kidney, liver and muscle by 13 days after dosing. These data collectively support a 13-day pre-slaughter withdrawal period.
A pivotal milk residue decline study was conducted in lactating dairy cattle. In this study, cows received a single injection of 3.0 mg CE/lb (6.6 mg CE/kg) BW. Ceftiofur residues in milk were less than tolerances at all time points after treatment. These data collectively support that no milk discard period is required for this product.
Two-Dose Residue Decline Studies
A pivotal tissue residue decline study was conducted in dairy cattle. In this study, cows received two injections of 3.0 mg CE/lb (6.6 mg CE/kg) BW with a 72 hour interval between injections. Ceftiofur residues in tissues were less than the tolerances for ceftiofur residues in the kidney by 13 days after the second dose. These data collectively continue to support a 13-day pre-slaughter withdrawal period after the last dose.
A pivotal milk residue decline study was conducted in lactating dairy cattle. In this study, cows received two injections of 3.0 mg CE/lb (6.6 mg CE/kg) BW with a 72 hour interval between injections. Milk residue decline data from this study supports that no milk discard period is required for this product.
STORAGE CONDITIONS
Store at controlled room temperature 20° to 25°C (68° to 77°F). Shake well before using. Contents should be used within 12 weeks after the first dose is removed.
HOW SUPPLIED
EXCEDE Sterile Suspension is available in the following package sizes:
-
-
NADA #141-209, Approved by FDA
Distributed by
Pharmacia & Upjohn Company
Division of Pfizer Inc, NY, NY 10017
www.EXCEDE.com or call 1-866-387-2287
Revised: December 2011
PRINCIPAL DISPLAY PANEL - 200 mg Bottle Label
EXCEDE
®
(Ceftiofur Crystalline Free Acid)
Sterile Suspension
100 mL
Equivalent to
200 mg/mL
ceftiofur
For subcutaneous injection in the posterior aspect of the ear where it attaches to the
head (base of the ear) in lactating dairy cattle. For subcutaneous injection in the
middle third of the posterior aspect of the ear or in the posterior aspect of the ear
where it attaches to the head (base of the ear) in beef and non-lactating dairy cattle.
Not for use in calves to be processed for veal. For intramuscular injection in the horse.
For the treatment of bovine respiratory disease (BRD) and for the treatment of bovine foot rot
(interdigital necrobacillosis) in beef, non-lactating dairy, and lactating dairy cattle. For the
control of respiratory disease in beef and non-lactating dairy cattle which are at high risk
of developing BRD. For the treatment of lower respiratory tract infections in horses
caused by susceptible strains of Streptococcus equi ssp. zooepidemicus.
For use in animals only
Caution: Federal (USA) law restricts this
drug to use by or on the order of a
licensed veterinarian.
NADA #141-209, Approved by FDA
Pfizer
PRINCIPAL DISPLAY PANEL - 200 mg Bottle Carton
EXCEDE
®
(Ceftiofur Crystalline Free Acid)
Sterile Suspension
Equivalent to 200 mg/mL ceftiofur
For subcutaneous injection in the posterior
aspect of the ear where it attaches to the head
(base of the ear) in lactating dairy cattle. For
subcutaneous injection in the middle third of
the posterior aspect of the ear or in the post-
erior aspect of the ear where it attaches to
the head (base of the ear) in beef and
non-lactating dairy cattle. Not for use in
calves to be processed for veal.
For intramuscular injection in
the horse.
For use in animals only
Caution:
Federal (USA) law restricts
this drug to use by or on
the order of a licensed
veterinarian.
100 mL Vial
NADA #141-209, Approved by FDA
Pfizer

Excedeceftiofur INJECTION, SUSPENSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||