EXTAVIA
Novartis Pharmaceuticals Corporation
HIGHLIGHTS OF PRESCRIBING INFORMATION INDICATIONS AND USAGEExtavia is an interferon beta indicated for the treatment of relapsing forms of multiple sclerosis to reduce the frequency of clinical exacerbations. Patients with multiple sclerosis in whom efficacy has been demonstrated include patients who have experienced a first clinical episode and have MRI features consistent with multiple sclerosis. (1) DOSAGE AND ADMINISTRATION For subcutaneous use only. (2) The recommended dose is 0.25 mg injected subcutaneously every other day. Generally, start at 0.0625 mg (0.25 mL) subcutaneously every other day, and increase over a six week period to 0.25 mg (1 mL) every other day. (2) Instruct patients in the use of aseptic technique when administering Extavia. (17.6) DOSAGE FORMS AND STRENGTHSLyophilized powder containing 0.3 mg of Interferon beta-1b, 15 mg Albumin (Human), USP, and 15 mg Mannitol, USP. (3).CONTRAINDICATIONSHistory of hypersensitivity to natural or recombinant interferon beta, Albumin (Human), USP, or any other component of the formulation. (4)WARNINGS AND PRECAUTIONS Depression and suicide: advise patients to immediately report any symptom of depression and/or suicidal ideation; consider discontinuation of Extavia if depression occurs. (5.1) Injection site necrosis: do not administer Extavia into affected area until it is fully healed; if multiple lesions occur, therapy should be discontinued until healing occurs. (5.2) Injection site reactions. (5.3) Anaphylaxis and other allergic reactions. (5.4) Flu-Like Symptom Complex. (5.5) Leukopenia: monitor CBC. (5.6, 5.8) Liver enzymes abnormalities: monitor liver function tests. (5.7, 5.8) Monitor thyroid function tests every 6 months in patients with history of thyroid dysfunction. (5.8) Side EffectsIn controlled studies with interferon beta-1b, the most common adverse reactions (at least 2% more than placebo) were: Lymphopenia, neutropenia, leukopenia, lymphadenopathy, headache, insomnia, incoordination, hypertension, dyspnea, abdominal pain, increased liver enzymes, rash, skin disorder, hypertonia, myalgia, urinary urgency, metrorrhagia, impotence, injection site reaction, asthenia, flu-like symptom complex, pain, fever, chills, peripheral edema, chest pain, malaise, and injection site necrosis (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSNo formal drug interaction studies have been conducted. (7)USE IN SPECIFIC POPULATIONS Pregnancy: Based on animal data, may cause fetal harm. (8.1) Nursing Mothers: use EXTAVIA with caution. (8.3) Pediatric Use: Safety and efficacy not established in patients under 18 years of age. (8.3) Geriatric Use: Safety and efficacy not established in patients age 65 years or older. (8.4)
FULL PRESCRIBING INFORMATION
EXTAVIA (Interferon beta-1b) is indicated for the treatment of relapsing forms of multiple sclerosis to reduce the frequency of clinical exacerbations. Patients with multiple sclerosis in whom efficacy has been demonstrated include patients who have experienced a first clinical episode and have MRI features consistent with multiple sclerosis.
The recommended dose of EXTAVIA is 0.25 mg injected subcutaneously every other day.
Generally, patients should be started at 0.0625 mg (0.25 mL) subcutaneously every other day, and increased over a six week period to 0.25 mg (1 mL) every other day (see Table 1).
| Recommended Titration |
EXTAVIA Dose |
Volume | |
| Weeks 1-2 | 25% | 0.0625 mg | 0.25 mL |
| Weeks 3-4 | 50% | 0.125 mg | 0.5 mL |
| Weeks 5-6 | 75% | 0.1875 mg | 0.75 mL |
| Week 7+ | 100% | 0.25 mg | 1 mL |
To reconstitute lyophilized EXTAVIA for injection, attach the prefilled syringe containing the diluent (Sodium Chloride, 0.54% Solution) to the EXTAVIA vial using the vial adapter. Slowly inject 1.2 mL of diluent into the EXTAVIA vial. Gently swirl the vial to dissolve the drug completely; do not shake. Foaming may occur during reconstitution or if the vial is swirled or shaken too vigorously. If foaming occurs, allow the vial to sit undisturbed until the foam settles. Visually inspect the reconstituted product before use; discard the product if it contains particulate matter or is discolored. Keeping the syringe and vial adapter in place, turn the assembly over so that the vial is on top. Withdraw the appropriate dose of EXTAVIA solution. Remove the vial from the vial adapter before injecting EXTAVIA. One mL of reconstituted EXTAVIA solution contains 0.25 mg of Interferon beta-1b/mL.
EXTAVIA is intended for use under the guidance and supervision of a physician. It is recommended that physicians or qualified medical personnel train patients in the proper technique for self-administering subcutaneous injections. Patients should be advised to rotate sites for subcutaneous injections (see Patient Counseling Information 17.6). Concurrent use of analgesics and/or antipyretics may help ameliorate flu-like symptoms on treatment days. EXTAVIA should be visually inspected for particulate matter and discoloration prior to administration.
EXTAVIA is supplied as a lyophilized powder containing 0.3 mg of Interferon beta-1b, 15 mg Albumin (Human), USP, and 15 mg Mannitol, USP. Drug is packaged in a clear glass, single-use vial (3 mL capacity). A pre-filled single-use syringe containing 1.2 mL of diluent (Sodium Chloride, 0.54% solution), two alcohol prep pads, and one vial adapter with attached 27 gauge needle are included for each vial of drug. EXTAVIA and the diluent are for single-use only. Unused portions should be discarded. Store at room temperature.
EXTAVIA is contraindicated in patients with a history of hypersensitivity to natural or recombinant interferon beta, Albumin (Human), USP, or any other component of the formulation.
EXTAVIA (Interferon beta-1b) should be used with caution in patients with depression, a condition that is common in people with multiple sclerosis. Depression and suicide have been reported to occur with increased frequency in patients receiving interferon compounds, including Interferon beta-1b. Patients treated with EXTAVIA should be advised to report immediately any symptoms of depression and/or suicidal ideation to their prescribing physicians. If a patient develops depression, cessation of EXTAVIA therapy should be considered.
In the four randomized controlled studies there were three suicides and eight suicide attempts among the 1532 patients in the Interferon beta-1b treated groups compared to one suicide and four suicide attempts among the 965 patients in the placebo groups.
Injection site necrosis (ISN) has been reported in 4% of patients in controlled clinical trials [ s ee Adverse Reactions (6.1)]. Typically, injection site necrosis occurs within the first four months of therapy, although post-marketing reports have been received of ISN occurring over one year after initiation of therapy. Necrosis may occur at a single or multiple injection sites. The necrotic lesions are typically three cm or less in diameter, but larger areas have been reported. Generally the necrosis has extended only to subcutaneous fat. However, there are also reports of necrosis extending to and including fascia overlying muscle. In some lesions where biopsy results are available, vasculitis has been reported. For some lesions debridement and, infrequently, skin grafting have been required.
As with any open lesion, it is important to avoid infection and, if it occurs, to treat the infection. Time to healing was varied depending on the severity of the necrosis at the time treatment was begun. In most cases healing was associated with scarring.
Some patients have experienced healing of necrotic skin lesions while Interferon beta-1b therapy continued; others have not. Whether to discontinue therapy following a single site of necrosis is dependent on the extent of necrosis. For patients who continue therapy with EXTAVIA after injection site necrosis has occurred, EXTAVIA should not be administered into the affected area until it is fully healed. If multiple lesions occur, therapy should be discontinued until healing occurs.
Patient understanding and use of aseptic self-injection techniques and procedures should be periodically reevaluated, particularly if injection site necrosis has occurred.
In controlled clinical trials, injection site reactions occurred in 78% of patients receiving Interferon beta-1b with injection site necrosis in 4%. Injection site inflammation (42%), injection site pain (16%), injection site hypersensitivity (4%), injection site necrosis (4%), injection site mass (2%), injection site edema (2%) and non-specific reactions were significantly associated with Interferon beta-1b treatment . The incidence of injection site reactions tended to decrease over time. Approximately 69% of patients experienced the event during the first three months of treatment, compared to approximately 40% at the end of the studies.
Anaphylaxis has been reported as a rare complication of Interferon beta-1b use. Other allergic reactions have included dyspnea, bronchospasm, tongue edema, skin rash and urticaria [ see Adverse Reactions (6.1 ) ] .
In controlled clinical trials, the rate of flu-like symptom complex was approximately 57%. The incidence decreased over time, with only 10% of patients reporting flu-like symptom complex at the end of the studies. The median duration of flu-like symptom complex in Study 1 was 7.5 days [see Clinical Studies (14)].
In controlled clinical trials, leukopenia was reported in 18% of patients receiving Interferon beta-1b, leading to a reduction of the dose of Interferon beta-1b in some patients [see Adverse Reactions (6.1)]. Monitoring of complete blood and differential white blood cell counts is recommended [see Warnings and Precautions (5. 8 )].
In controlled clinical trials, elevations of SGPT to greater than five times baseline value were reported in 12% of patients receiving Interferon beta-1b, and increase of SGOT to greater than five times baseline value were reported in 4% of patients receiving Interferon beta-1b, leading to dose-reduction or discontinuation of treatment in some patients [see A dverse R eactions (6.1)]. Monitoring of liver function tests is recommended [see Warnings and Precautions (5. 8 )].
In addition to those laboratory tests normally required for monitoring patients with multiple sclerosis, complete blood and differential white blood cell counts, platelet counts and blood chemistries, including liver function tests, are recommended at regular intervals (one, three, and six months) following introduction of EXTAVIA therapy, and then periodically thereafter in the absence of clinical symptoms. Thyroid function tests are recommended every six months in patients with a history of thyroid dysfunction or as clinically indicated. Patients with myelosuppression may require more intensive monitoring of complete blood cell counts, with differential and platelet counts.
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) also is considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for albumin.
In all studies, the most serious adverse reactions with Interferon beta-1b were depression, suicidal ideation and injection site necrosis (see Warnings and Precautions). The incidence of depression of any severity was approximately 30% in both Interferon beta-1b-treated patients and placebo-treated patients. Anaphylaxis and other allergic reactions have been reported in patients using Interferon beta-1b [see Warnings and Precautions (5. 4 )]. The most commonly reported adverse reactions were lymphopenia (lymphocytes<1500/mm3), injection site reaction, asthenia, flu-like symptom complex, headache, and pain. The most frequently reported adverse reactions resulting in clinical intervention (e.g., discontinuation of Interferon beta-1b, adjustment in dosage, or the need for concomitant medication to treat an adverse reaction symptom) were depression, flu-like symptom complex, injection site reactions, leukopenia, increased liver enzymes, asthenia, hypertonia, and myasthenia.
Because clinical trials are conducted under widely varying conditions and over varying lengths of time, adverse reaction rates observed in the clinical trials of Interferon beta-1b cannot be directly compared to rates in clinical trials of other drugs, and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
The data described below reflect exposure to Interferon beta-1b in the four placebo controlled trials of 1407 patients with MS treated with 0.25 mg or 0.16 mg/m2, including 1261 exposed for greater than one year. The population encompassed an age range from 18 – 65 years. Sixty-four percent (64%) of the patients were female. The percentages of Caucasian, Black, Asian, and Hispanic patients were 94.8%, 3.5%, 0.1%, and 0.7%, respectively.
The safety profiles for Interferon beta-1b-treated patients with SPMS and RRMS were similar. Clinical experience with Interferon beta-1b in other populations (patients with cancer, HIV positive patients, etc.) provides additional data regarding adverse reactions; however, experience in non-MS populations may not be fully applicable to the MS population.
Table 2 enumerates adverse events and laboratory abnormalities that occurred among all patients treated with 0.25 mg or 0.16 mg/m2 Interferon beta-1b every other day for periods of up to three years in the four placebo controlled trials (Study 1-4) at an incidence that was at least 2.0% more than that observed in the placebo patients (System Organ Class, MedDRA v. 8.0).
| System Organ Class MedDRA v. 8.0 #
Adverse Reaction |
Placebo (n=965) | Interferon beta-1b (n=1407) |
| Blood and lymphatic system disorders | ||
| Lymphocytes count decreased (< 1500/mm3) x | 66% | 86% |
| Absolute neutrophil count decreased (< 1500/mm3) x | 5% | 13% |
| White blood cell count decreased (< 3000/mm3) x | 4% | 13% |
| Lymphadenopathy | 3% | 6% |
| Nervous system disorders | ||
| Headache | 43% | 50% |
| Insomnia | 16% | 21% |
| Incoordination | 15% | 17% |
| Vascular disorders | ||
| Hypertension | 4% | 6% |
| Respiratory, thoracic and mediastinal disorders | ||
| Dyspnea | 3% | 6% |
| Gastrointestinal disorders | ||
| Abdominal pain | 11% | 16% |
| Hepatobiliary disorders | ||
| Alanine aminotransferase increased(SGPT > 5 times baseline)x | 4% | 12% |
| Aspartate aminotransferase increased(SGOT > 5 times baseline)x | 1% | 4% |
| Skin and subcutaneous tissue disorders | ||
| Rash | 15% | 21% |
| Skin disorder | 8% | 10% |
| Musculoskeletal and connective tissue disorders | ||
| Hypertonia | 33% | 40% |
| Myalgia | 14% | 23% |
| Renal and urinary disorders | ||
| Urinary urgency | 8% | 11% |
| Reproductive system and breast disorders | ||
| Metrorrhagia* | 7% | 9% |
| Impotence** | 6% | 8% |
| General disorders and administration site conditions | ||
| Injection site reaction (various kinds ) 0 | 26% | 78% |
| Asthenia | 48% | 53% |
| Flu-like symptoms (complex)§ | 37% | 57% |
| Pain | 35% | 42% |
| Fever | 19% | 31% |
| Chills | 9% | 21% |
| Peripheral edema | 10% | 12% |
| Chest pain | 6% | 9% |
| Malaise | 3% | 6% |
| Injection site necrosis | 0% | 4% |
|
# except for "injection site reaction (various kinds)o" and "flu-like symptom complex§" the most appropriate MedDRA term is used to describe a certain reaction and its synonyms and related conditions. x laboratory abnormality * pre-menopausal women ** men o "Injection site reaction (various kinds)" comprises all adverse events occurring at the injection site (except injection site necrosis), i.e., the following terms: injection site reaction, injection site hemorrhage, injection site hypersensitivity, injection site inflammation, injection site mass, injection site pain, injection site edema and injection site atrophy. § "Flu-like symptom complex" denotes flu syndrome and/or a combination of at least two AEs from fever, chills, myalgia, malaise, sweating. |
||
Laboratory Abnormalities
In the four clinical trials, leukopenia was reported in 18% and 6% of patients in Interferon beta-1b- and placebo-treated groups, respectively. No patients were withdrawn or dose reduced for neutropenia in Study 1. Three percent (3%) of patients in Studies 2 and 3 experienced leukopenia and were dose-reduced. Monitoring of complete blood and differential white blood cell counts is recommended [see Warnings and Precautions (5.6, 5.8)].
Other abnormalities included increase of SGPT to greater than five times baseline value (12%), and increase of SGOT to greater than five times baseline value (4%). In Study 1, two patients were dose reduced for increased hepatic enzymes; one continued on treatment and one was ultimately withdrawn. In Studies 2 and 3, 1.5% of Interferon beta-1b patients were dose-reduced or interrupted treatment for increased hepatic enzymes. In Study 4, 1.7% of patients were withdrawn from treatment due to increased hepatic enzymes, two of them after a dose reduction. In Studies 1-4, nine (0.6%) patients were withdrawn from treatment with Interferon beta-1b for any laboratory abnormality, including four (0.3%) patients following dose reduction. Monitoring of liver function tests is recommended [see Warnings and Precautions (5.7, 5.8)].
The following adverse events have been observed during postmarketing experience with Interferon beta-1b and are classified within body system categories:
Blood and lymphatic system disorders: Anemia, Thrombocytopenia
Endocrine disorders: Hypothyroidism, Hyperthyroidism, Thyroid dysfunction
Metabolism and nutrition disorders: Hypocalcemia, Hyperuricemia, Triglyceride increased, Anorexia, Weight decrease
Psychiatric disorders: Confusion, Depersonalization, Emotional lability
Nervous system disorders: Ataxia, Convulsion, Paresthesia, Psychotic symptoms
Cardiac disorders: Cardiomyopathy
Vascular disorders: Deep vein thrombosis, Pulmonary embolism
Respiratory, thoracic and mediastinal disorders: Bronchospasm, Pneumonia
Gastrointestinal disorders: Pancreatitis, Vomiting
Hepatobiliary disorders: Hepatitis, Gamma GT increased
Skin and subcutaneous tissue disorders: Pruritus, Skin discoloration, Urticaria
Renal and urinary disorders: Urinary tract infection, Urosepsis
General disorders and administration site conditions: Fatal capillary leak syndrome*.
*The administration of cytokines to patients with a pre-existing monoclonal gammopathy has been associated with the development of this syndrome.
As with all therapeutic proteins, there is a potential for immunogenicity. Serum samples were monitored for the development of antibodies to Interferon beta-1b during Study 1 [see Clinical Studies (14)]. In patients receiving 0.25 mg every other day 56/124 (45%) were found to have serum neutralizing activity at one or more of the time points tested. In Study 4 [see Clinical Studies (14)], neutralizing activity was measured every 6 months and at end of study. At individual visits after start of therapy, activity was observed in 16.5% up to 25.2% of the Interferon beta-1b treated patients. Such neutralizing activity was measured at least once in 75 (29.9%) out of 251 Interferon beta-1b patients who provided samples during treatment phase; of these, 17 (22.7%) converted to negative status later in the study.
Based on all the available evidence, the relationship between antibody formation and clinical safety or efficacy is not known.
These data reflect the percentage of patients whose test results were considered positive for antibodies to Interferon beta-1b using a biological neutralization assay that measures the ability of immune sera to inhibit the production of the interferon-inducible protein, MxA. Neutralization assays are highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of neutralizing activity in an assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Interferon beta-1b with the incidence of antibodies to other products may be misleading.
Anaphylactic reactions have rarely been reported with the use of Interferon beta-1b [ s ee Warnings and Precautions (5.4)].
No formal drug interaction studies have been conducted with Interferon beta-1b. In the placebo controlled studies in MS, corticosteroids or ACTH were administered for treatment of relapses for periods of up to 28 days in patients (N=664) receiving Interferon beta-1b.
Pregnancy Category C: There are no adequate and well-controlled studies of Interferon beta-1b in pregnant women; however, spontaneous abortions while on treatment were reported in four patients participating in the Interferon beta-1b RRMS clinical trial. Interferon beta-1b should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
When Interferon beta-1b (doses ranging from 0.028 to 0.42 mg/kg) was administered to pregnant rhesus monkeys throughout the period of organogenesis (gestation days 20 to 70), a dose-related abortifacient effect was observed. The low effect dose is approximately 3 times the recommended human dose of 0.25 mg on a body surface are (mg/m2) basis. A no-effect dose for embryo-fetal developmental toxicity in rhesus monkeys was not established.
It is not known whether Interferon beta-1b is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Interferon beta-1b, a decision should be made to either discontinue nursing or discontinue the drug, taking into account the importance of drug to the mother.
Safety and efficacy in pediatric patients have not been established.
Clinical studies of Interferon beta-1b did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients.
Safety of doses higher than 0.25 mg every other day has not been adequately evaluated. The maximum amount of Interferon beta-1b that can be safely administered has not been determined.
EXTAVIA® (Interferon beta-lb) is a purified, sterile, lyophilized protein product produced by recombinant DNA techniques. Interferon beta-1b is manufactured by bacterial fermentation of a strain of Escherichia coli that bears a genetically engineered plasmid containing the gene for human interferon betaser17. The native gene was obtained from human fibroblasts and altered in a way that substitutes serine for the cysteine residue found at position 17. Interferon beta-1b has 165 amino acids and an approximate molecular weight of 18,500 daltons. It does not include the carbohydrate side chains found in the natural material. EXTAVIA contains the same active ingredients as other Interferon beta-1b products. For this reason, these products should not be given concomitantly.
The specific activity of EXTAVIA is approximately 32 million international units (IU)/mg Interferon beta-lb. Each vial contains 0.3 mg of Interferon beta-lb. The unit measurement is derived by comparing the antiviral activity of the product to the World Health Organization (WHO) reference standard of recombinant human interferon beta. Mannitol, USP and Albumin (Human), USP (15 mg each/vial) are added as stabilizers.
Lyophilized EXTAVIA is a sterile, white to off-white powder, for subcutaneous injection after reconstitution with the diluent supplied (Sodium Chloride, 0.54% Solution).
The mechanism of action of Interferon beta-1b in patients with multiple sclerosis is unknown.
Interferons (IFNs) are a family of naturally occurring proteins, produced by eukaryotic cells in response to viral infection and other biologic agents. Four major groups of interferons have been distinguished: alpha, beta, gamma and lambda. Interferons-alpha and -beta comprise the Type I interferons, interferon-gamma is the sole Type II interferon, and interferon-lambda is designated as Type III interferon. Type I interferons have considerably overlapping but also distinct biologic activities. The bioactivities of IFNs are mediated by their interactions with specific receptors found on the surfaces of human cells. Differences in bioactivites induced by IFNs likely reflect divergences in the signal transduction process induced by IFN-receptor binding.
Interferon beta-1b receptor binding induces the expression of proteins that are responsible for the pleiotropic bioactivities of Interferon beta-1b. A number of these proteins (including neopterin, β2-microglobulin, MxA protein, and IL-10) have been measured in blood fractions from Interferon beta-1b-treated patients and Interferon beta-1b-treated healthy volunteers. Immunomodulatory effects of Interferon beta-1b include the enhancement of suppressor T cell activity, reduction of pro-inflammatory cytokine production, down-regulation of antigen presentation, and inhibition of lymphocyte trafficking into the central nervous system. It is not known if these effects play an important role in the observed clinical activity of Interferon beta-1b in multiple sclerosis (MS).
Because serum concentrations of Interferon beta-1b are low or not detectable following subcutaneous administration of 0.25 mg or less of Interferon beta-1b, pharmacokinetic information in patients with MS receiving the recommended dose of Interferon beta-1b is not available. Following single and multiple daily subcutaneous administrations of 0.5 mg Interferon beta-1b to healthy volunteers (N=12), serum Interferon beta-1b concentrations were generally below 100 IU/mL. Peak serum Interferon beta-1b concentrations occurred between one to eight hours, with a mean peak serum interferon concentration of 40 IU/mL. Bioavailability, based on a total dose of 0.5 mg Interferon beta-1b given as two subcutaneous injections at different sites, was approximately 50%.
After intravenous administration of Interferon beta-1b (0.006 mg to 2.0 mg), similar pharmacokinetic profiles were obtained from healthy volunteers (N=12) and from patients with diseases other than MS (N=142). In patients receiving single intravenous doses up to 2.0 mg, increases in serum concentrations were dose proportional. Mean serum clearance values ranged from 9.4 mL/min•kg -1 to 28.9 mL/min•kg-1 and were independent of dose. Mean terminal elimination half-life values ranged from 8.0 minutes to 4.3 hours and mean steady-state volume of distribution values ranged from 0.25 L/kg to 2.88 L/kg. Three-times-a-week intravenous dosing for two weeks resulted in no accumulation of Interferon beta-1b in sera of patients. Pharmacokinetic parameters after single and multiple intravenous doses of Interferon beta-1b were comparable.
Following every other day subcutaneous administration of 0.25 mg Interferon beta-1b in healthy volunteers, biologic response marker levels (neopterin, β2- microglobulin, MxA protein, and the immunosuppressive cytokine, IL-10) increased significantly above baseline six-twelve hours after the first Interferon beta-1b dose. Biologic response marker levels peaked between 40 and 124 hours and remained elevated above baseline throughout the seven-day (168-hour) study. The relationship between serum Interferon beta-1b levels or induced biologic response marker levels and the clinical effects of Interferon beta-1b in multiple sclerosis is unknown.
Carcinogenesis: Interferon beta-1b has not been tested for its carcinogenic potential in animals.
Mutagenesis: Interferon beta-1b was not genotoxic in the in vitro Ames bacterial test or the in vitro chromosomal aberration assay in human peripheral blood lymphocytes. Interferon beta-1b treatment of mouse BALBc-3T3 cells did not result in increased transformation frequency in an in vitro model of tumor transformation.
Impairment of fertility: Administration of Interferon beta-1b (doses of up to 0.33 mg/kg) to normally cycling female rhesus monkeys had no apparent adverse effects on either menstrual cycle duration or associated hormonal profiles (progesterone and estradiol) when administered over three consecutive menstrual cycles. The highest dose tested is approximately 30 times the recommended human dose of 0.25 mg on a body surface area (mg/m2) basis. The potential for other effects on fertility or reproductive performance was not evaluated.
The clinical effects of Interferon beta-1b were studied in four randomized, multicenter, double-blind, placebo-controlled studies in patients with multiple sclerosis.
The effectiveness of Interferon beta-1b in relapsing-remitting MS (Study 1) was evaluated in a double blind, multiclinic, randomized, parallel, placebo controlled clinical investigation of two years’ duration. The study enrolled MS patients, aged 18 to 50, who were ambulatory (EDSS of ≤ 5.5), exhibited a relapsing-remitting clinical course, met Poser’s criteria1 for clinically definite and/or laboratory supported definite MS and had experienced at least two exacerbations over two years preceding the trial without exacerbation in the preceding month. Patients who had received prior immunosuppressant therapy were excluded.
An exacerbation was defined as the appearance of a new clinical sign/symptom or the clinical worsening of a previous sign/symptom (one that had been stable for at least 30 days) that persisted for a minimum of 24 hours.
Patients selected for study were randomized to treatment with either placebo (N=123), 0.05 mg of Interferon beta-1b (N=125), or 0.25 mg of Interferon beta-1b (N=124) self-administered subcutaneously every other day. Outcome based on the 372 randomized patients was evaluated after two years.
Patients who required more than three 28-day courses of corticosteroids were removed from the study. Minor analgesics (acetaminophen, codeine), antidepressants, and oral baclofen were allowed ad libitum, but chronic nonsteroidal anti-inflammatory drug (NSAID) use was not allowed.
The primary protocol-defined outcome measures were 1) frequency of exacerbations per patient and 2) proportion of exacerbation free patients. A number of secondary clinical and magnetic resonance imaging (MRI) measures were also employed. All patients underwent annual T2 MRI imaging and a subset of 52 patients at one site had MRIs performed every six weeks for assessment of new or expanding lesions.
The study results are shown in Table 3.
| Efficacy Parameters | Treatment Groups | Statistical Comparisons p-value | |||||
| Primary End Points | Placebo N=123 |
0.05 mg N=125 |
0.25 mg N=124 |
Placebo vs 0.05 mg |
0.05 mg vs 0.25 mg |
Placebo vs 0.25 mg |
|
| Annual exacerbation rate | 1.31 | 1.14 | 0.90 | 0.005 | 0.113 | 0.0001 | |
| Proportion of exacerbation-free patients† | 16% | 18% | 25% | 0.609 | 0.288 | 0.094 | |
| Exacerbation frequency per patient | 0† 1 2 3 4 ≥5 |
20 32 20 15 15 21 |
22 31 28 15 7 16 |
29 39 17 14 9 8 |
0.151 | 0.077 | 0.001 |
| Secondary Endpoints †† | |||||||
| Median number of months to first on-study exacerbation | 5 | 6 | 9 | 0.299 | 0.097 | 0.010 | |
| Rate of moderate or severe exacerbations per year | 0.47 | 0.29 | 0.23 | 0.020 | 0.257 | 0.001 | |
| Mean number of moderate or severe exacerbation days per patient | 44.1 | 33.2 | 19.5 | 0.229 | 0.064 | 0.001 | |
| Mean change in EDSS score‡ at endpoint | 0.21 | 0.21 | -0.07 | 0.995 | 0.108 | 0.144 | |
| Mean change in Scripps score‡‡ at endpoint | -0.53 | -0.50 | 0.66 | 0.641 | 0.051 | 0.126 | |
| Median duration in days per exacerbation | 36 | 33 | 35.5 | ND | ND | ND | |
| % change in mean MRI lesion area at endpoint | 21.4% | 9.8% | -0.9% | 0.015 | 0.019 | 0.0001 | |
| ND Not done † 14 exacerbation free patients (0 from placebo, six from 0.05 mg, and eight from 0.25 mg) dropped out of the study before completing six months of therapy. These patients are excluded from this analysis. †† Sequelae and Functional Neurologic Status, both required by protocol, were not analyzed individually but are included as a function of the EDSS. ‡ EDSS scores range from 1-10, with higher scores reflecting greater disability ‡‡ Scripps neurologic rating scores range from 0-100, with smaller scores reflecting greater disability. |
|||||||
Of the 372 RRMS patients randomized, 72 (19%) failed to complete two full years on their assigned treatments.
Over the two-year period, there were 25 MS-related hospitalizations in the 0.25 mg Interferon beta-1b-treated group compared to 48 hospitalizations in the placebo group. In comparison, non-MS hospitalizations were evenly distributed among the groups, with 16 in the 0.25 mg Interferon beta-1b group and 15 in the placebo group. The average number of days of MS-related steroid use was 41 days in the 0.25 mg Interferon beta-1b group and 55 days in the placebo group (p=0.004).
MRI data were also analyzed for patients in this study. A frequency distribution of the observed percent changes in MRI area at the end of two years was obtained by grouping the percentages in successive intervals of equal width. Figure 1 displays a histogram of the proportions of patients, which fell into each of these intervals. The median percent change in MRI area for the 0.25 mg group was -1.1%, which was significantly smaller than the 16.5% observed for the placebo group (p=0.0001).
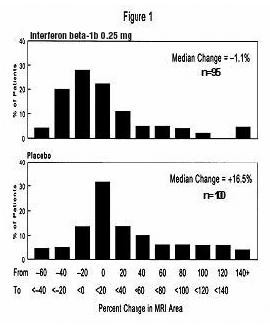
Patients treated with Interferon beta-1b demonstrated a lower number of newly active lesions during the course of the study. A significant difference between Interferon beta-1b and placebo was not seen in the absolute change in T2 lesion volume during the course of the study.
Safety and efficacy of treatment with Interferon beta-1b beyond three years are not known.
1. Poser CM, et al. Ann Neurol 1983; 13(3): 227-231.
2. Kurtzke JF. Neurology 1983; 33(11): 1444-1452.
The reconstituted product contains no preservative. Before reconstitution with diluent, store EXTAVIA at room temperature 25°C (77°F). Excursions of 15° to 30°C (59° to 86°F) are permitted. After reconstitution, if not used immediately, the product should be refrigerated and used within three hours. Do not freeze.
EXTAVIA is supplied as a lyophilized powder containing 0.3 mg of Interferon beta-1b, 15 mg Albumin (Human), USP, and 15 mg Mannitol, USP. Drug is packaged in a clear glass, single-use vial (3 mL capacity). A pre-filled single-use syringe containing 1.2 mL of diluent (Sodium Chloride, 0.54% solution), two alcohol prep pads, and one vial adapter with attached 27 gauge needle are included for each vial of drug. EXTAVIA and the diluent are for single-use only. Unused portions should be discarded. Store at room temperature.
15 blister units, 0.3 mg/vial…………………….…….NDC 0078-0569-12
All patients should be instructed to carefully read the supplied EXTAVIA Medication Guide. Patients should be cautioned not to change the dose or schedule of administration without medical consultation.
Advise patients that depression and suicidal ideation have been reported during the use of Interferon beta-1b. Advise patients of the symptoms of depression or suicidal ideation, and instruct patients to report them immediately to their physician [see Warnings and Precautions (5.1)].
Advise patients that injection site reactions occur in most patients treated with Interferon beta-1b, and that injection site necrosis may occur at one or multiple sites. Instruct patients to promptly report any break in the skin, which may be associated with blue-black discoloration, swelling, or drainage of fluid from the injection site, prior to continuing their EXTAVIA therapy [see Warnings and Precautions (5.2, 5.3)].
Advise patients of the symptoms of allergic reactions and anaphylaxis, and instruct patients to seek immediate medical attention if these symptoms occur [see Warnings and Precautions (5.4)].
Patients should be informed that flu-like symptoms are common following initiation of therapy with Interferon beta-1b. In controlled clinical trials, antipyretics and analgesics were permitted for relief of these symptoms. In addition, gradual dose titration during initiation of Interferon beta-1b treatment may reduce flu-like symptoms [see Warnings and Precautions (5.5) and D osage A nd A dministration (2)].
Advise patients that EXTAVIA should not be used during pregnancy unless the potential benefit justifies the potential risk to the fetus [see Use in Special Population (8.1)] .
Patients should be instructed in the use of aseptic technique when administering EXTAVIA. Appropriate instruction for reconstitution of EXTAVIA and methods of self-injection should be provided, including careful review of the EXTAVIA Medication Guide. The first injection should be performed under the supervision of an appropriately qualified health care professional.
Patients should be cautioned against the re-use of needles or syringes and instructed in safe disposal procedures. A puncture resistant container for disposal of used needles and syringes should be supplied to the patient along with instructions for safe disposal of full containers.
Patients should be advised of the importance of rotating areas of injection with each dose, to minimize the likelihood of severe injection site reactions, including necrosis or localized infection, (see Choose an Injection Site section of the Medication Guide).
Manufactured by:
Bayer HealthCare Pharmaceuticals Inc.
Montville, NJ 07045
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, NJ 07936
For
Novartis Pharmaceuticals Corporation
East Hanover, NJ 07936
U.S. License No. 1244
MEDICATION GUIDE
EXTAVIA ( ex tā vee uh ) Interferon beta-1b
Read the Medication Guide that comes with EXTAVIA before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your doctor about your medical condition or your treatment.
What is the most important information I should know about EXTAVIA?
EXTAVIA and other Interferon beta-1b medicines will not cure multiple sclerosis (MS) but have been shown to decrease the number of flare-ups of the disease. Interferon beta-1b medicines, including EXTAVIA, can cause serious side effects. Before you start to take EXTAVIA, you should talk to your doctor about the possible risks and benefits of EXTAVIA.
EXTAVIA may cause serious side effects, including:
- Depression
- Allergic reactions
- Injection site problems
These serious side effects are described below.
1. Depression. Some people who take interferon medicines, including EXTAVIA, become seriously depressed (feeling sad or sinking spirits). Some people have thoughts about killing themselves (suicidal thoughts) or try to kill themselves. Depression is not uncommon in people with multiple sclerosis.
Before you start to take EXTAVIA, tell your doctor if you ever had any mental illness, including depression, or if you take any medicines for depression.
- While you take EXTAVIA, if you feel noticeably sadder or helpless, or feel like hurting yourself or others, you should tell a family member or friend right away and call your doctor as soon as possible. You may need to stop taking EXTAVIA.
2. Allergic reactions. Some people who take Interferon beta-1b medicines, including EXTAVIA, have severe allergic reactions which can lead to trouble breathing and swallowing. Significant swelling of the mouth and tongue may occur with these severe allergic reactions. These reactions can happen quickly. Allergic reactions can happen after your first dose of EXTAVIA or may not happen until after you have taken EXTAVIA many times. Less severe allergic reactions such as rash, itching, skin bumps or minor swelling of the mouth and tongue can also happen. If you think you are having an allergic reaction, stop taking EXTAVIA right away and call your doctor.
3. Injection site problems. Interferon beta-1b medicines, including EXTAVIA, may cause redness, pain or swelling at the place where an injection was given (injection site). Serious skin reactions can happen in some people, including skin infections or areas of severe damage to skin and tissue below the skin (necrosis). These reactions can happen anywhere you inject EXTAVIA.
Call your doctor right away if you have any of these signs of a serious problem at any of your injection sites:
- the area is swollen and painful
- the area looks infected, and does not heal within a few days
- the area has fluid draining from it
- you notice any breaks in your skin or blue-black skin discoloration of your skin along with a break in your skin.
Most skin reactions are not serious, but you may need medical treatment if you develop a serious skin reaction. In most cases healing was associated with scarring.
If multiple lesions occur, therapy should be discontinued until healing occurs.
What is EXTAVIA?
EXTAVIA is a man-made form of a protein called beta interferon. EXTAVIA is similar to certain interferon proteins that are produced in the body.
EXTAVIA is used to treat relapsing forms of multiple sclerosis (MS). It will not cure your MS but may decrease the number of flare-ups of the disease. MS is a life-long disease that affects your nervous system by destroying the protective covering (myelin) that surrounds your nerve fibers. The way EXTAVIA works in MS is not known.
Who should not take EXTAVIA?
Do not take EXTAVIA if you :
- have had an allergic reaction such as trouble breathing, skin flushing, or hives, with another interferon beta product, or to human albumin.
- are allergic to any of the ingredients in EXTAVIA. See the end of this Medication Guide for a list of the ingredients in EXTAVIA.
What should I tell my doctor before taking EXTAVIA?
Tell your doctor about all your medical conditions, including if you have:
- or had depression, anxiety (feeling uneasy, nervous, or fearful), or trouble sleeping
- liver problems
- thyroid problems
- blood problems, such as bleeding or bruising easily, and low red blood cells (anemia) or low white blood cells
- are pregnant or plan to become pregnant. If you become pregnant while you take EXTAVIA, stop taking EXTAVIA and call your doctor right away. Interferon beta-1b medicines, including EXTAVIA, may cause you to lose your pregnancy (miscarriage) or may cause harm to your unborn child. You and your doctor will need to decide whether the possible benefit of taking EXTAVIA is more important than the possible risks to your unborn child.
- are breastfeeding or plan to breastfeed. It is not known if EXTAVIA passes into your milk. You and your doctor should decide if you will breastfeed or take EXTAVIA. You should not do both without talking with your doctor.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
How should I take EXTAVIA?
- Take EXTAVIA exactly as prescribed by your doctor. Do not change your dose unless told to by your doctor.
- If your doctor decides that you or a caregiver may be able to give your injections of EXTAVIA at home, your doctor or nurse should instruct you on the right way to prepare and inject EXTAVIA. Do not try to inject EXTAVIA yourself until you have been instructed by your doctor or nurse the right way to prepare and give the injections.
- EXTAVIA is given by injection under the skin (subcutaneous injection) every other day.
- If you miss a dose of EXTAVIA, take your next dose as soon as you remember or are able to take it. Take your next injection about 2 days after that dose. If you are not sure when you should take your next dose, call your doctor.
-
Do not take EXTAVIA two days in a row (consecutive days).
- Call your doctor right away if you take more than your prescribed dose of EXTAVIA, or take it two days in a row.
-
Always use a new, unopened, vial of EXTAVIA and syringe for each injection. Throw away any unused medicine. Do not reuse any vials, syringes, or needles.
- It is important for you to change your injection site each time you inject EXTAVIA. This will lessen the chance of you having a serious skin reaction at the site where you inject EXTAVIA.
- Avoid injecting EXTAVIA into an area of skin that is sore, red, infected or has other problems.
- See the end of this Medication Guide for detailed Patient Instructions for Use for information about how to mix and inject EXTAVIA the right way.
What are the possible side effects of EXTAVIA?
EXTAVIA can cause serious side effects. See “What is the most important information I should know about EXTAVIA?”.
Common side effects of EXTAVIA include:
-
Flu-like symptoms. Most people have flu-like symptoms (fever, chills, sweating, muscle aches and tiredness) when taking EXTAVIA. These symptoms may lessen or go away over time. Talk to your doctor about whether you should take a non-prescription medicine for pain, or to lower fever before or after you take your dose of EXTAVIA.
-
Liver problems. EXTAVIA may affect your liver function. Your doctor will do blood tests to check for these problems while you take EXTAVIA. Tell your doctor if you have any of these symptoms of a liver problem:
- yellowing of the skin and whites of the eyes
- easy bruising
- right-sided stomach area (abdominal) pain
- yellowing of the skin and whites of the eyes
-
Blood problems. You may have a decrease in the amount of certain blood cells, including white blood cells (blood cells that fight infection), red blood cells (blood cells that carry oxygen to body tissues), or platelets (blood cells that help you form blood clots). If this decrease is severe, your body may be less able to fight infections, you may feel tired or sluggish, or you may bruise or bleed easily.
-
Thyroid problems. Your thyroid function may change. Symptoms of changes in the function of your thyroid include feeling cold or hot much of the time, or change in your weight (gain or loss) without a change in your diet or amount of exercise you are getting.
-
Asthenia.
You may feel excessively or unusually fatigued. Talk to your doctor about your fatigue if it is persistent and bothersome to you.
-
Headache. You may develop headaches. You should tell your doctor if you experience headaches while taking EXTAVIA, and you should make a plan with your doctor for monitoring your headaches. Talk to your doctor about whether you should take an additional medicine for the headaches.
- Pain. You may experience pain while taking EXTAVIA. Talk to your doctor about whether you should take a non-prescription medicine for pain and keep your doctor informed about any changes in the pain you experience.
You should discuss with your doctor the need for blood testing to monitor for these problems. Your doctor will arrange for testing your blood at regular intervals to help detect blood, thyroid, liver, or other problems that may develop. These blood tests will be needed even if you do not have any symptoms.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of EXTAVIA. For more information ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store EXTAVIA?
- Before mixing, store EXTAVIA at room temperature 25oC (77oF). Storage at temperatures between 15o to 30oC (59o to 86oF) for brief periods of time are acceptable.
- After mixing, if you can not inject EXTAVIA right away, refrigerate the medicine and inject it within 3 hours. If you can not inject the mixed medicine within 3 hours, do not use it. Follow the information in the Patient Instructions for Use section “Dispose of used needles, syringes, and vials” for the right way to throw away the syringe with the unused medicine, and needle.
- Do not freeze EXTAVIA.
Keep EXTAVIA and all medicines out of the reach of children.
General information about EXTAVIA
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use EXTAVIA for a condition for which it has not been prescribed. Do not give EXTAVIA to other people even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about EXTAVIA. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about EXTAVIA that is written for health professionals. For more information go to the web site www.EXTAVIA.com or call the EXTAVIA toll-free medical information line at 1-888-669-6682.
What are the ingredients in EXTAVIA?
Active ingredient: interferon beta-1b
Inactive ingredients: mannitol, albumin (human).
The diluent contains sodium chloride solution.
EXTAVIA Patient Instructions for Use
If your doctor decides that you or a caregiver may be able to give your injections of EXTAVIA at home, your doctor or nurse should instruct you on the right way to prepare and inject EXTAVIA. To lower your risk of infection, it is important that you follow the technique that your doctor or nurse discussed with you to prepare and inject EXTAVIA. Do not try to inject EXTAVIA yourself until you have been shown by your doctor or nurse the right way to prepare and give the injections.
It is important for you to read, understand, and follow these instructions. Call your doctor if you or your caregiver has any questions about the right way to prepare or inject EXTAVIA.
Important safety information
- Do not leave the blister pack containing EXTAVIA where others might tamper with it.
- Keep the blister pack containing EXTAVIA out of the reach of children.
- Do not open the blister pack or take out any of the items until right before you are ready to use them.
- Do not use EXTAVIA if the seal on the vial is broken. If the seal is broken, the product may not be safe for you to use.
- Do not use EXTAVIA after the expiration date shown on the blister pack label or box (Figure 1). If it has expired, return the entire pack to the pharmacy.
Figure 1
- Do not use any of the items in the blister pack more than one time. See the section at the end of this leaflet, “Dispose of used syringes, needles, and vials”. Throw away any open and unused medicine.
Gather your supplies.
You will need the following supplies to get ready to give your injection of EXTAVIA:
- A blister pack containing the following items (Figure 2)
- a vial of EXTAVIA
- a prefilled syringe of diluent (Sodium Chloride, 0.54% solution)
- a vial adapter with a 27-gauge needle attached (in its own container)
- two (2) alcohol wipes
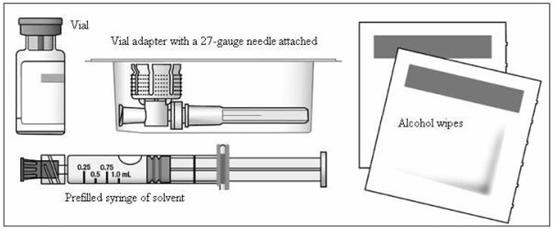
Figure 2
- a dry cotton ball and gauze
- a sharps disposal container (Figure 3). See the section “Dispose of used syringes, needles, and vials.”
Figure 3
Prepare for self-injection
- Wash your hands well with soap and water.
- Open the blister pack by peeling off the label and take out all the items. Make sure the blister pack containing the vial adapter is sealed. Check to make sure the rubber cap on the diluent syringe is firmly attached.
- Turn the blister pack over, and place the vial in the well (vial holder) and place the prefilled syringe in the U-shaped trough (Figure 4).
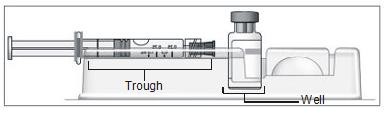
Figure 4
Mix EXTAVIA
4. Remove the EXTAVIA vial from the well and take the cap off the vial (Figure 5).
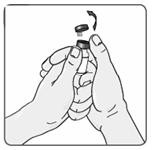
Figure 5
5. Place the vial back in the vial holder.
6. Use an alcohol wipe to clean the top of the vial (Figure 6). Wipe in one direction only.

Figure 6
7. Leave the alcohol wipe on top of the vial until step 9 below.
8. Peel the label off the container with the vial adapter in it, but do not remove the vial adapter. The vial adapter is sterile, so do not touch it.
9. Remove the alcohol wipe from the top of the vial. Pick up the container that holds the vial adapter. Turn over the container keeping the vial adaptor inside. Put the adapter on top of the vial. Push down on the adapter until it pierces the rubber top of the vial and snaps in place (Figure 7). Lift the container off the vial adapter.
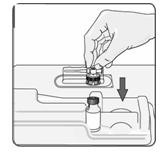
Figure 7
10. Remove the rubber cap from the prefilled syringe using a twist and pull motion (Figure 8). Throw away the rubber cap.
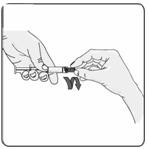
Figure 8
11. Remove the vial from the vial holder by grasping the vial. Do not touch any part of the vial adapter. Be careful not to pull the vial adapter off the top of the vial.
12. Connect the prefilled syringe of diluent to the vial adapter by turning clockwise and tighten carefully (Figure 9).
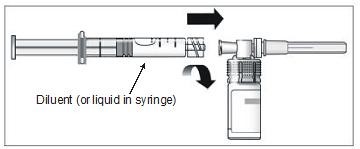
Figure 9
13. Slowly push the plunger of the prefilled syringe all the way in. This will push all of the liquid from the syringe into the vial (Figure 10). Continue to hold the plunger while you mix EXTAVIA with the liquid from the syringe. If you do not hold the plunger in it may return to its original position after you let go.
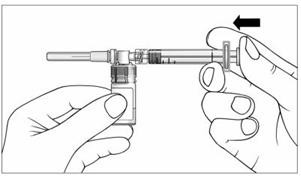
Figure 10
14. Gently swirl the vial to completely dissolve the white powder (EXTAVIA). Do not shake. Shaking and even gentle mixing can cause foaming of the medicine. If there is foam, let the vial sit until the foam settles.
15. After the powder dissolves, look closely at the solution in the vial. Do not use the solution if it is not clear or colorless, or if it contains particles.
The injection should be given right away after you mix EXTAVIA and let any foam in the solution settle. If you must wait for any reason before giving yourself the injection, you may refrigerate the medicine after you mix it. But you should use it within three hours.
16. With your thumb still pushing the plunger, turn the syringe and vial, so that the vial is on top (Figure 11).
17. Slowly pull the plunger back to withdraw the entire contents of the vial into the syringe.
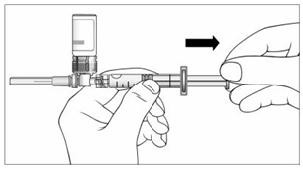
Figure 11
18. Turn the syringe so that the needle end is pointing up. Remove any air bubbles by tapping the outside of the syringe with your fingers (Figure 12). Slowly push the plunger to the 1 mL mark on the syringe or to the mark that matches the amount of EXTAVIA prescribed by your doctor. If too much solution is pushed back into the vial, return to step 16.
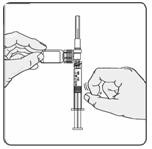
Figure 12
19. Remove the vial adapter and the vial from the syringe by twisting the vial adapter (Figure 13).
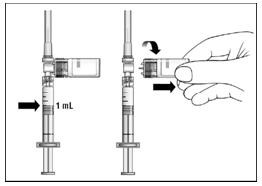
Figure 13
Choose an Injection Site
- EXTAVIA is injected under the skin and into the fat layer between the skin and the muscles (subcutaneous tissue). The best areas for injection are where the skin is loose and soft and away from the joints, nerves, and bones. Do not use the area near your navel (belly button) or waistline. If you are very thin, use only the thigh or outer surface of the arm for injection.
- Choose a different site each time you give yourself an injection. Figure 14 shows different areas for giving injections. Do not inject in the same area for two injections in a row. Keep a record of your injections to help make sure you change (rotate) your injection sites. If there are any sites that are difficult for you to reach, you can ask someone who has been trained to give the injection to you.
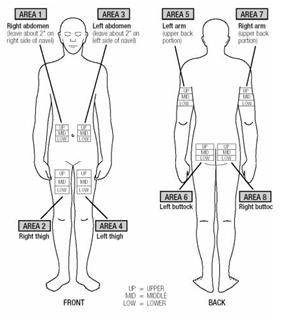
Figure 14
- Do not inject EXTAVIA in a site where the skin is red, bruised, infected, or scabbed, has broken open, or has lumps, bumps, or pain. Tell your doctor if you find skin conditions like the ones mentioned here or any other unusual looking areas where you have been given injections.
Injecting EXTAVIA
20. Using a circular motion, clean the injection site with an alcohol wipe, starting at the injection site and moving outward (Figure 15). Let the skin area air dry.
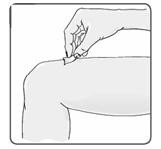
Figure 15
21. Remove the cap from the needle (Figure 16).
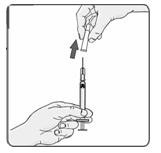
Figure 16
22. Gently pinch the skin around the site with your thumb and forefinger of the other hand (Figure 17). Insert the needle straight up and down into your skin at a 90˚ angle with a quick, dart-like motion.
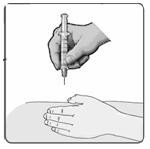
Figure 17
23. Once the needle is in your skin, slowly pull back on the plunger. If blood appears in the syringe it means that you have entered a blood vessel. Do not inject EXTAVIA. Withdraw the needle. Throw away the syringe and needle in your puncture-proof container. Do not use the same syringe or any of the other supplies that you used for this injection. Repeat the above steps to prepare your dose using a new blister pack. Choose and clean a new injection site.
24. If no blood appears in the syringe, slowly push the plunger all the way in until the syringe is empty (Figure 18). Remove the needle from the skin; then place a dry cotton ball or gauze pad over the injection site. Gently massage the injection site for a few minutes with the dry cotton ball or gauze pad. Throw away the syringe in your puncture-proof disposal container.
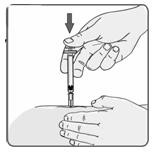
Figure 18
Dispose of used syringes, needles, and vials
- To prevent needle-stick injury and spread of infection, do not try to re-cap the needle.
- Place used needles, syringes, and vials in a closeable, puncture-resistant container. You may use a sharps container (such as a red biohazard container), a hard plastic container (such as a detergent bottle), or a metal container (such as an empty coffee can). Do not use glass or clear plastic containers. Ask your doctor for instructions on the right way to throw away (dispose of) the container. There may be state and local laws about how you should throw away used needles and syringes.
- Do not throw used needles, syringes, or vials in your household trash or recycle. Throw away any unused medicine. Do not save any unused EXTAVIA for a future dose.
Keep the disposal container, needles, syringes, and vials of EXTAVIA out of the reach of children.
Manufactured by:
Bayer HealthCare Pharmaceuticals Inc.
Montville, NJ 07045
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, NJ 07936
For
Novartis Pharmaceuticals Corporation
East Hanover, NJ 07936
U.S. License No. 1244
This Medication Guide has been approved by the U.S. Food and Drug Administration
© Novartis
T2012-69/T2012-70
March 2012/March 2012
PRINCIPAL DISPLAY PANEL
Package Label – 0.3 mg per vial
Rx Only NDC 0078-0569-12
EXTAVIA®
(interferon beta-1b)
0.3 mg per vial
For subcutaneous use only
Dispense the enclosed Medication Guide to each patient.
Each blister pack contains:
- one 1.2 mL prefilled single use sodium chloride 0.54% solution diluent syringe
- one single use vial for reconstitution containing Extavia
- one vial adapter with attached 27-guage needle
- two alcohol prep pads
Dispense in this unit-of-use container.
15 single use blister packs

EXTAVIAInterferon beta-1b KIT
| |||||||||||||||||||||||||||||||||||||||||||||