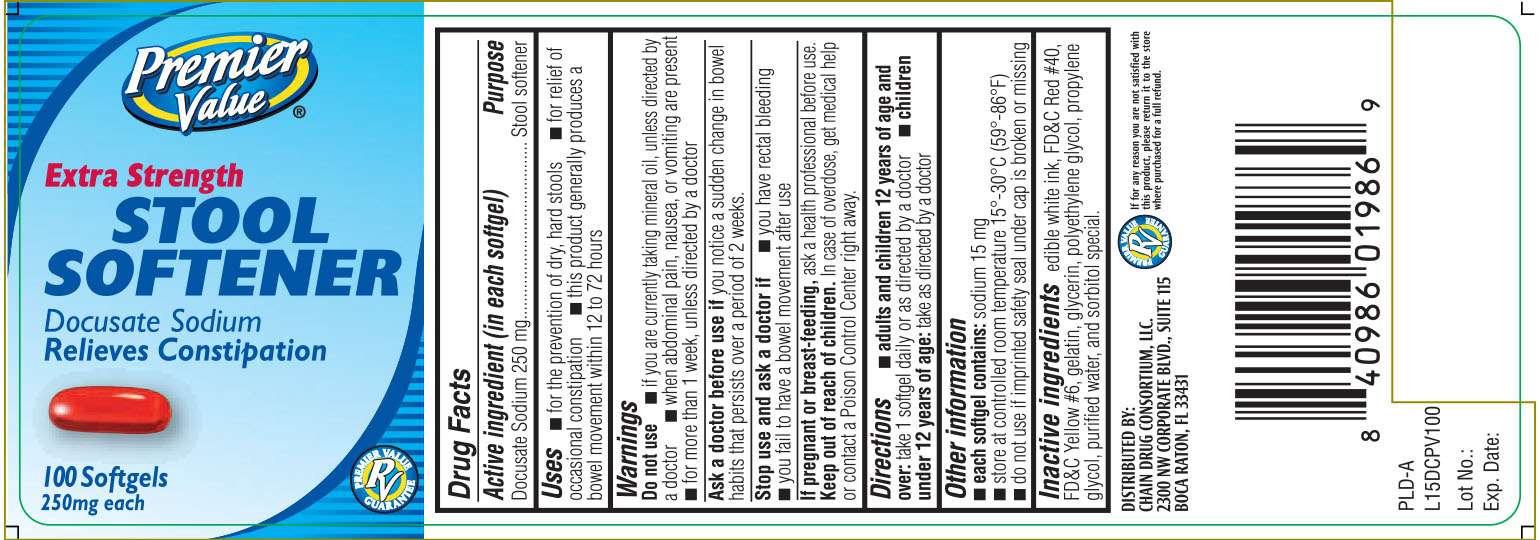
EXTRA STRENGTH STOOL SOFTENER
Chain Drug Consortium, LLC (Premier Value)
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Docusate Sodium 250 mg
Stool softener
- for the prevention of dry, hard stools
- for relief of occasional constipation.
- this product generally produces a bowel movement within 12 to 72 hours.
- if you are taking mineral oil, unless directed by a doctor
- when abdominal pain, nausea, or vomiting are present
- for more than 1 week, unless directed by a doctor
you notice a sudden change in bowel habits that persists over a period of 2 weeks
- you have rectal bleeding
- you fail to have a bowel movement after use
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
-
adults and children over 12 years of age and over: take 1 softgel daily or as directed by a doctor
- children under 12 years of age: take as directed by a doctor
- each softgel contains: Sodium 15 mg
- store at controlled room temperature 15o - 30o C (59o- 86o F)
- do not use if imprinted safety seal under cap is broken or missing
edible white ink, FD&C Red No# 40, FD&C Yellow #6, gelatin, glycerin, polyethylene glycol, propylene glycol, purified water and sorbitol special.
Extra Strength
STOOL SOFTENER
Docusate Sodium
Relieves Constipation
DISTRIBUTED BY:
CHAIN DRUG CONSORTIUM, LLC
2300 NW CORPORATE BLVD., SUITE 115
BOCA RATON, FL 33431
Product Label
EXTRA STRENGTH STOOL SOFTENERDOCUSATE SODIUM CAPSULE, LIQUID FILLED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||