Eye Drops
Rugby Laboratories Inc.
Bausch & Lomb Incorporated
Eye Drops Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Eye Drops Uses
- Warnings
- Directions
- Eye Drops Other information
- Inactive ingredients
- Questions or comments?
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Tetrahydrozoline HCl 0.05%
Purpose
Redness Reliever
Eye Drops Uses
- relieves redness of the eye due to minor eye irritations
Warnings
Do not use if solution changes color or becomes cloudy
Ask a doctor before use if you have
narrow angle glaucoma
When using this product
- do not touch tip of container to any surface to avoid contamination
- overuse may produce increased redness of the eye
- pupils may become enlarged temporarily
Stop use and ask a doctor if
- you experience eye pain, changes in vision, continued redness or irritation of the eye
- condition worsens or persists for more than 72 hours
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- instill 1 to 2 drops in the affected eye(s) up to four times daily
Eye Drops Other information
- store at 15° - 30°C (59° - 86°F)
- keep tightly closed
- replace cap after use
Inactive ingredients
boric acid, edetate disodium, sodium borate, sodium chloride, purified water.
Hydrochloric acid and/or sodium hydroxide may be added to adjust pH.
PRESERVATIVE: benzalkonium chloride 0.01%
Questions or comments?
Call 1-800-645-2158
9 am - 5 pm ET, Monday-Friday.
Serious side effects associated with use of this product may be reported to this number.
Package/Label Principal Display Panel
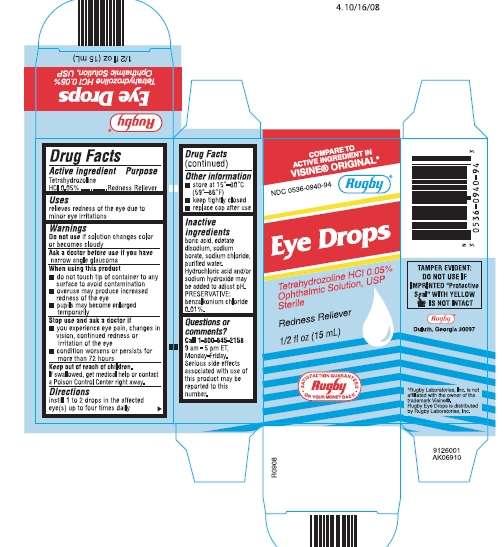
NDC 0536-0940-94
Rugby®
COMPARED TO ACTIVE INGREDIENT IN VISINE® ORIGINAL*
Eye Drops
Tetrahydrozoline HCl 0.05%
Ophthalmic Solution, USP
Sterile
Redness Reliever
1/2 fl oz (15 mL)
Rugby®
Eye DropsTetrahydrozoline HCl SOLUTION/ DROPS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||