Famciclovir
HIGHLIGHTS OF PRESCRIBING INFORMATION INDICATIONS AND USAGE Famciclovir, a prodrug of penciclovir, is a nucleoside analogue DNA polymerase inhibitor indicated for: Immunocompetent Adult Patients (1.1) • Herpes labialis (cold sores) o Treatment of recurrent episodes • Genital herpes o Treatment of recurrent episodes o Suppressive therapy of recurrent episodes • Herpes zoster (shingles) HIV-Infected Adult Patients (1.2) • Treatment of recurrent episodes of orolabial or genital herpes Limitation of Use (1.3) The efficacy and safety of famciclovir have not been established for:• Patients Patients with renal impairment: Adjust dose based on creatinine clearance (2.3) DOSAGE FORMS AND STRENGTHSTablets : 125mg, 250mg, 500mg (3) CONTRAINDICATIONSKnown hypersensitivity to the product, its components, or Denavir (penciclovir cream) (4) WARNINGS AND PRECAUTIONSAcute renal failure: May occur in patients with underlying renal disease who receive higher than recommended doses of famciclovir for their level of renal function. Reduce dosage in patients with renal impairment (2.3, 8.6)Side EffectsThe most common adverse events reported in at least one indication by >10% of adult patients are headache and nausea. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Macleods Pharma USA Inc. at 1-888-943-3210 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONSProbenecid: May increase penciclovir levels. Monitor for evidence of penciclovir toxicity (7.2) USE IN SPECIFIC POPULATIONS Nursing mothers: Famciclovir tablets should not be used in nursing mothers unless the potential benefits outweigh the potential risks associated with treatment.(8.3)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 INDICATIONS & USAGE
- 2 DOSAGE & ADMINISTRATION
- 3 DOSAGE FORMS & STRENGTHS
- 4 FAMCICLOVIR CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 FAMCICLOVIR ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 FAMCICLOVIR DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
1 INDICATIONS & USAGE
1.1 Immunocompetent Adult Patient
Herpes labialis (cold sores): Famciclovir is indicated for the treatment of recurrent herpes labialis.
Genital herpes:
Recurrent episodes: Famciclovir is indicated for the treatment of recurrent episodes of genital herpes. The efficacy of Famciclovir when initiated more than 6 hours after onset of symptoms or lesions has not been established.
Suppressive therapy: Famciclovir is indicated for chronic suppressive therapy of recurrent episodes of genital herpes. The efficacy and safety of famciclovir for the suppression of recurrent genital herpes beyond 1 year have not been established.
Herpes zoster (shingles): Famciclovir is indicated for the treatment of herpes zoster. The efficacy of famciclovir when initiated more than 72 hours after onset of rash has not been established.
1.2 HIV-Infected Adult Patients
Recurrent orolabial or genital herpes: Famciclovir is indicated for the treatment of recurrent episodes of orolabial or genital herpes in HIV-infected adults. The efficacy of famciclovir when initiated more than 48 hours after onset of symptoms or lesions has not been established.
1.3 Limitation of Use
The efficacy and safety of famciclovir have not been established for:
• Patients <18 years of age
• Patients with first episode of genital herpes
• Patients with ophthalmic zoster
• Immunocompromised patients other than for the treatment of recurrent orolabial or genital herpes in HIV-infected patient.
2 DOSAGE & ADMINISTRATION
Famciclovir Tablets may be taken with or without food.
2.1 Dosing Recommendation in Immunocompetent Adult Patients
Herpes labialis (cold sores): The recommended dosage of famciclovir for the treatment of recurrent herpes labialis is 1500 mg as a single dose. Therapy should be initiated at the first sign or symptom of herpes labialis (e.g., tingling, itching, burning, pain, or lesion).
Genital herpes:
Recurrent episodes: The recommended dosage of famciclovir for the treatment of recurrent episodes of genital herpes is 1000 mg twice daily for 1 day. Therapy should be initiated at the first sign or symptom of a recurrent episode (e.g., tingling, itching, burning, pain, or lesion).
Suppressive therapy: The recommended dosage of famciclovir for chronic suppressive therapy of recurrent episodes of genital herpes is 250 mg twice daily.
Herpes zoster (shingles): The recommended dosage of famciclovir for the treatment of herpes zoster is 500 mg every 8 hours for 7 days. Therapy should be initiated as soon as herpes zoster is diagnosed.
2.2 Dosing Recommendation in HIV-Infected Adult Patients
Recurrent orolabial or genital herpes: The recommended dosage of famciclovir for the treatment of recurrent orolabial or genital herpes in HIV-infected patients is 500 mg twice daily for 7 days. Therapy should be initiated at the first sign or symptom of a recurrent episode (e.g., tingling, itching, burning, pain, or lesion).
2.3 Dosing Recommendation in Patients with Renal Impairment
Dosage recommendations for adult patients with renal impairment are provided in Table 1 [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
| Indication and Normal Dosage Regimen |
Creatinine Clearance (mL/min.) |
Adjusted Dosage Regimen Dose (mg) |
Dosing Interval |
| Single-Day Dosing Regimens | |||
| Recurrent Genital Herpes 1000 mg every 12 hours for 1 day |
≥60 | 1000 | every 12 hours for 1 day |
| 40 to 59 | 500 | every 12 hours for 1 day | |
| 20 to 39 | 500 | single dose | |
| <20 | 250 | single dose | |
HD |
250 | single dose following dialysis |
|
| Recurrent Herpes Labialis 1500 mg single dose |
≥60 | 1500 | single dose |
| 40 to 59 | 750 | single dose | |
| 20 to 39 | 500 | single dose | |
| <20 | 250 | single dose | |
HD |
250 | single dose following dialysis |
|
| Multiple-Day Dosing Regimens | |||
| Herpes Zoster 500 mg every 8 hours |
≥60 | 500 | every 8 hours |
| 40 to 59 | 500 | every 12 hours | |
| 20 to 39 | 500 | every 24 hours | |
| <20 | 250 | every 24 hours | |
HD |
250 | following each dialysis | |
| Suppression of Recurrent Genital Herpes 250 mg every 12 hours |
≥40 | 250 | every 12 hours |
| 20 to 39 | 125 | every 12 hours | |
| <20 | 125 | every 24 hours | |
HD |
125 | following each dialysis | |
| Recurrent Orolabial and Genital Herpes in HIV-Infected Patients 500 mg every 12 hours |
≥40 | 500 | every 12 hours |
| 20 to 39 | 500 | every 24 hours | |
| <20 | 250 | every 24 hours | |
HD |
250 | following each dialysis | |
3 DOSAGE FORMS & STRENGTHS
Famciclovir tablets are available in three strengths:
• 125 mg: White to off white, round film-coated, biconvex, engraved with “ML 67” on one side and plain on the other side.
• 250 mg: White to off white, round film-coated, biconvex, engraved with “ML 70” on one side and plain on the other side.
• 500 mg: White to off white, oval film-coated, biconvex, engraved with “ML 72” on one side and plain on the other side.
4 CONTRAINDICATIONS
Famciclovir tablets are contraindicated in patients with known hypersensitivity to the product, its components, or Denavir® (penciclovir cream).
5 WARNINGS AND PRECAUTIONS
Acute renal failure: Cases of acute renal failure have been reported in patients with underlying renal disease who have received inappropriately high doses of famciclovir for their level of renal function. Dosage reduction is recommended when administering famciclovir tablets to patients with renal impairment [see Dosage and Administration (2.3), Use in Specific Populations (8.6)].
6 ADVERSE REACTIONS
Acute renal failure is discussed in greater detail in other sections of the label [see Warnings and Precautions (5)].
The most common adverse events reported in at least 1 indication by >10% of adult patients treated with famciclovir are headache and nausea.
6.1 Clinical Trials Experience in Adult Patient
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Immunocompetent patients: The safety of famciclovir has been evaluated in active- and placebo-controlled clinical studies involving 816 famciclovir treated patients with herpes zoster (famciclovir , 250 mg three times daily to 750 mg three times daily); 163 famciclovir treated patients with recurrent genital herpes ( famciclovir, 1000 mg twice daily); 1,197 patients with recurrent genital herpes treated with famciclovir as suppressive therapy (125 mg once daily to 250 mg three times daily) of which 570 patients received famciclovir (open-labeled and/or double-blind) for at least 10 months; and 447 famciclovir treated patients with herpes labialis ( famciclovir, 1500 mg once daily or 750 mg twice daily). Table 2 lists selected adverse events.
|
|
Incidence |
|||||||
|
|
Herpes Zoster  |
Recurrent Genital Herpes  |
Genital Herpes- Suppression  |
Herpes Labialis |
||||
|
Event |
Famciclovir |
Placebo |
Famciclovir |
Placebo |
Famciclovir |
Placebo |
Famciclovir |
Placebo |
|
|
(n=273) |
(n=146) |
(n=163) |
(n=166) |
(n=458) |
(n=63) |
(n=447) |
(n=254) |
|
|
% |
% |
% |
% |
% |
% |
% |
% |
|
Nervous System |
||||||||
|
Headache |
22.7 |
17.8 |
13.5 |
5.4 |
39.3 |
42.9 |
8.5 |
6.7 |
|
Paresthesia |
2.6 |
0.0 |
0.0 |
0.0 |
0.9 |
0.0 |
0.0 |
0.0 |
|
Migraine |
0.7 |
0.7 |
0.6 |
0.6 |
3.1 |
0.0 |
0.2 |
0.0 |
|
Gastrointestinal |
||||||||
|
Nausea |
12.5 |
11.6 |
2.5 |
3.6 |
7.2 |
9.5 |
2.2 |
3.9 |
|
Diarrhea |
7.7 |
4.8 |
4.9 |
1.2 |
9.0 |
9.5 |
1.6 |
0.8 |
|
Vomiting |
4.8 |
3.4 |
1.2 |
0.6 |
3.1 |
1.6 |
0.7 |
0.0 |
|
Flatulence |
1.5 |
0.7 |
0.6 |
0.0 |
4.8 |
1.6 |
0.2 |
0.0 |
|
Abdominal Pain |
1.1 |
3.4 |
0.0 |
1.2 |
7.9 |
7.9 |
0.2 |
0.4 |
|
Body as a Whole |
||||||||
|
Fatigue |
4.4 |
3.4 |
0.6 |
0.0 |
4.8 |
3.2 |
1.6 |
0.4 |
|
Skin and Appendages |
||||||||
|
Pruritus |
3.7 |
2.7 |
0.0 |
0.6 |
2.2 |
0.0 |
0.0 |
0.0 |
|
Rash |
0.4 |
0.7 |
0.0 |
0.0 |
3.3 |
1.6 |
0.0 |
0.0 |
|
Reproductive Female |
||||||||
|
Dysmenorrhea |
0.0 |
0.7 |
1.8 |
0.6 |
7.6 |
6.3 |
0.4 |
0.0 |
Table 3 selected laboratory abnormalities in genital herpes suppression trialsTable 3 selected laboratory abnormalities in genital herpes suppression trials
|
Parameter |
Famciclovir (n = 660) % |
Placebo (n = 210)  % |
|
Anemia (<0.8 x NRL) |
0.1 |
0.0 |
|
Leukopenia (<0.75 x NRL) |
1.3 |
0.9 |
|
Neutropenia (<0.8 x NRL) |
3.2 |
1.5 |
|
AST (SGOT) (>2 x NRH) |
2.3 |
1.2 |
|
ALT (SGPT) (>2 x NRH) |
3.2 |
1.5 |
|
Total Bilirubin (>1.5 x NRH) |
1.9 |
1.2 |
|
Serum Creatinine (>1.5 x NRH) |
0.2 |
0.3 |
|
Amylase (>1.5 x NRH) |
1.5 |
1.9 |
|
Lipase (>1.5 x NRH) |
4.9 |
4.7 |
NRH = Normal Range High.
NRL = Normal Range Low.
6.2 Postmarketing Experience
The adverse events listed below have been reported during post-approval use of famciclovir tablets. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
Blood and lymphatic system disorders: Thrombocytopenia
Hepatobiliary disorders: Abnormal liver function tests, cholestatic jaundice
Nervous system disorders: Dizziness, somnolence
Psychiatric disorders: Confusion (including delirium, disorientation, and confusional state occurring predominantly in the elderly), hallucinations
Skin and subcutaneous tissue disorders: Urticaria, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, angiodema (e.g. face, eyelid, periorbital and pharyngeal edema)
7 DRUG INTERACTIONS
7.1 Potential for famciclovir to Affect Other Drugs
The steady-state pharmacokinetics of digoxin were not altered by concomitant administration of multiple doses of famciclovir (500 mg three times daily). No clinically significant effect on the pharmacokinetics of zidovudine, its metabolite zidovudine glucuronide, or emtricitabine was observed following a single oral dose of 500 mg famciclovir coadministered with zidovudine or emtricitabine.
An in vitro study using human liver microsomes suggests that famciclovir is not an inhibitor of CYP3A4 enzymes.
7.2 Potential for Other Drugs to Affect Penciclovir
No clinically significant alterations in penciclovir pharmacokinetics were observed following single-dose administration of 500 mg famciclovir after pre-treatment with multiple doses of allopurinol, cimetidine, theophylline, zidovudine, promethazine, when given shortly after an antacid (magnesium and aluminum hydroxide), or concomitantly with emtricitabine. No clinically significant effect on penciclovir pharmacokinetics was observed following multiple-dose (three times daily) administration of famciclovir (500 mg) with multiple doses of digoxin.
Concurrent use with probenecid or other drugs significantly eliminated by active renal tubular secretion may result in increased plasma concentrations of penciclovir.
The conversion of 6-deoxy penciclovir to penciclovir is catalyzed by aldehyde oxidase. Interactions with other drugs metabolized by this enzyme and/or inhibiting this enzyme could potentially occur. Clinical interaction studies of famciclovir with cimetidine and promethazine, in vitro inhibitors of aldehyde oxidase, did not show relevant effects on the formation of penciclovir. Raloxifene, a potent aldehyde oxidase inhibitor in vitro, could decrease the formation of penciclovir. However, a clinical drug-drug interaction study to determine the magnitude of interaction between penciclovir and raloxifene has not been conducted.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy category B: After oral administration, famciclovir (prodrug) is converted to penciclovir (active drug). There are no adequate and well-controlled studies of famciclovir or penciclovir use in pregnant women. No adverse effects on embryofetal development were observed in animal reproduction studies using famciclovir and penciclovir at doses higher than the maximum recommended human dose (MRHD) and human exposure. Because animal reproduction studies are not always predictive of human response, famciclovir should be used during pregnancy only if needed.
In animal reproduction studies, pregnant rats and rabbits received oral famciclovir at doses (up to 1000 mg/kg/day) that provided 2.7 to 10.8 times (rats) and 1.4 to 5.4 times (rabbits) the human systemic exposure based on AUC. No adverse effects were observed on embryo-fetal development. In other studies, pregnant rats and rabbits received intravenous famciclovir at doses (360 mg/kg/day) 1.5 to 6 times (rats) and (120 mg/kg/day) 1.1 to 4.5 times (rabbits) or penciclovir at doses (80/mg/kg/day) 0.3 to 1.3 times (rats) and (60 mg/kg/day) 0.5 to 2.1 times (rabbits) the MRHD based on body surface area comparisons. No adverse effects were observed on embryo-fetal development.
8.3 Nursing Mothers
It is not known whether famciclovir (prodrug) or penciclovir (active drug) are excreted in human milk. Following oral administration of famciclovir to lactating rats, penciclovir was excreted in breast milk at concentrations higher than those seen in the plasma. There are no data on the safety of famciclovir in infants. Famciclovir should not be used in nursing mothers unless the potential benefits are considered to outweigh the potential risks associated with treatment.
8.4 Pediatric Use
The efficacy and safety of famciclovir tablets have not been established in pediatric patients. Labeling describing additional clinical pharmacokinetic studies in pediatric patients (ages of 1 month to < 12 years) is approved for Novartis Pharmaceuticals Corporation's Famvir® Tablets. However, due to Novartis Pharmaceuticals Corporation's marketing exclusivity rights, a description of those clinical pharmacokinetic studies is not approved for this famciclovir tablet product.
8.5 Geriatric Use
Of 816 patients with herpes zoster in clinical studies who were treated with famciclovir, 248 (30.4%) were ≥65 years of age and 103 (13%) were ≥75 years of age. No overall differences were observed in the incidence or types of adverse events between younger and older patients. Of 610 patients with recurrent herpes simplex (type 1 or type 2) in clinical studies who were treated with famciclovir, 26 (4.3%) were >65 years of age and 7 (1.1%) were >75 years of age. Clinical studies of famciclovir in patients with recurrent genital herpes did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently compared to younger subjects.
No famciclovir dosage adjustment based on age is recommended unless renal function is impaired [see Dosage and Administration (2.3), Clinical Pharmacology (12.3)]. In general, appropriate caution should be exercised in the administration and monitoring of famciclovir in elderly patients reflecting the greater frequency of decreased renal function and concomitant use of other drugs.
8.6 Patients with Renal Impairment
Apparent plasma clearance, renal clearance, and the plasma-elimination rate constant of penciclovir decreased linearly with reductions in renal function. After the administration of a single 500-mg famciclovir oral dose (n=27) to healthy volunteers and to volunteers with varying degrees of renal impairment (CLCR ranged from 6.4 to 138.8 mL/min), the following results were obtained (Table 4):
| Table 4 Pharmacokinetic Parameters of Peniclovir in Subjects with Different Degrees of Renal Impairment | ||||
|
Parameter (mean ± S.D.) |
CLCR
 (mL/min.) (n=15) |
CLCR 40-59 (mL/min.) (n=5) |
CLCR 20-39 (mL/min.) (n=4) |
CLCR <20 (mL/min.) (n=3) |
| CLCR (mL/min) | 88.1 ± 20.6 | 49.3 ± 5.9 | 26.5 ± 5.3 | 12.7 ± 5.9 |
| CLCR (L/hr) | 30.1 ± 10.6 | 13.0 ± 1.3 |
4.2 ± 0.9 | 1.6 ± 1.0 |
CL/F |
66.9 ± 27.5 | 27.3 ± 2.8 | 12.8 ± 1.3 | 5.8 ± 2.8 |
| Half-life (hr) | 2.3 ± 0.5 | 3.4 ± 0.7 | 6.2 ± 1.6 | 13.4 ± 10.2 |
8.7 Patients with Renal Impairment
Mild or moderate hepatic impairment (chronic hepatitis [n=6], chronic ethanol abuse [n=8], or primary biliary cirrhosis [n=1]) had no effect on the extent of availability (AUC) of penciclovir following a single dose of 500 mg famciclovir. However, there was a 44% decrease in penciclovir mean maximum plasma concentration (Cmax) and the time to maximum plasma concentration (tmax) was increased by 0.75 hours in patients with hepatic impairment compared to normal volunteers. No dosage adjustment is recommended for patients with mild or moderate hepatic impairment. The pharmacokinetics of penciclovir has not been evaluated in patients with severe hepatic impairment. Conversion of famciclovir to the active metabolite penciclovir may be impaired in these patients resulting in a lower penciclovir plasma concentrations, and thus possibly a decrease of efficacy of famciclovir (see section 12 Clinical Pharmacology).
10 OVERDOSAGE
Appropriate symptomatic and supportive therapy should be given. Penciclovir is removed by hemodialysis.
11 DESCRIPTION
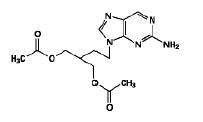
The active ingredient in famciclovir tablets is famciclovir, an orally administered prodrug of the antiviral agent penciclovir. Chemically, famciclovir is known as 2-[2-(2-amino-9H-purin-9-yl)ethyl]-1,3-propanediol diacetate. Its molecular formula is C14H19N5O4; its molecular weight is 321.3. It is a synthetic acyclic guanine derivative and has the following structure:
Famciclovir is a white to pale yellow solid. It is freely soluble in acetone and methanol, and sparingly soluble in ethanol and isopropanol. At 25°C famciclovir is freely soluble (>25% w/v) in water initially, but rapidly precipitates as the sparingly soluble (2% to 3% w/v) monohydrate. Famciclovir is not hygroscopic below 85% relative humidity. Partition coefficients are: octanol/water (pH 4.8) P=1.09 and octanol/phosphate buffer (pH 7.4) P=2.08.
Famciclovir tablets contain 125 mg, 250 mg or 500 mg of famciclovir, together with the following inactive ingredients: hydroxypropyl cellulose, hypromellose, anhydrous lactose, magnesium stearate, polyethylene glycols, sodium starch glycolate and titanium dioxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Famciclovir is an orally administered prodrug of the antiviral agent penciclovir [see Clinical Pharmacology (12.4)].
12.3 Pharmacokinetics
Famciclovir is the diacetyl 6-deoxy analog of the active antiviral compound penciclovir. Following oral administration famciclovir undergoes rapid and extensive metabolism to penciclovir and little or no famciclovir is detected in plasma or urine. Penciclovir is predominantly eliminated unchanged by the kidney. Therefore, the dose of famciclovir needs to be adjusted in patients with different degrees of renal impairment [see Dosage and Administration (2.3)].
Pharmacokinetics in adults:
Absorption and Bioavailability: The absolute bioavailability of penciclovir is 77 ± 8% as determined following the administration of a 500 mg famciclovir oral dose and a 400 mg penciclovir intravenous dose to 12 healthy male subjects.
Penciclovir concentrations increased in proportion to dose over a famciclovir dose range of 125 mg to 1000 mg administered as a single dose. Table 5 shows the mean pharmacokinetic parameters of penciclovir after single administration of famciclovir to healthy male volunteers.
| Dose | AUC (0-inf) (mcg hr/mL) |
Cmax (mcg/mL) |
Tmax (h) |
| 125 mg | 2.24 | 0.8 | 0.9 |
| 250 mg | 4.48 | 1.6 | 0.9 |
| 500 mg | 8.95 | 3.3 | 0.9 |
| 1000 mg | 17.9 | 6.6 | 0.9 |
Following oral single-dose administration of 500-mg famciclovir to seven patients with herpes zoster, the AUC (mean ± SD), Cmax, and Tmax were 12.1±1.7 mcg hr/mL, 4.0±0.7 mcg/mL, and 0.7±0.2 hours, respectively. The AUC of penciclovir was approximately 35% greater in patients with herpes zoster as compared to healthy volunteers. Some of this difference may be due to differences in renal function between the two groups.
There is no accumulation of penciclovir after the administration of 500-mg famciclovir three times daily for 7 days.
Penciclovir Cmax decreased approximately 50% and Tmax was delayed by 1.5 hours when a capsule formulation of famciclovir was administered with food (nutritional content was approximately 910 Kcal and 26% fat). There was no effect on the extent of availability (AUC) of penciclovir. There was an 18% decrease in Cmax and a delay in Tmax of about 1 hour when famciclovir was given 2 hours after a meal as compared to its administration 2 hours before a meal. Because there was no effect on the extent of systemic availability of penciclovir, famciclovir tablets can be taken without regard to meals.
Distribution: The volume of distribution (Vdβ) was 1.08±0.17 L/kg in 12 healthy male subjects following a single intravenous dose of penciclovir at 400 mg administered as a 1-hour intravenous infusion. Penciclovir is <20% bound to plasma proteins over the concentration range of 0.1 to 20 mcg/mL. The blood/plasma ratio of penciclovir is approximately 1.
Metabolism: Following oral administration, famciclovir is deacetylated and oxidized to form penciclovir. Metabolites that are inactive include 6-deoxy penciclovir, monoacetylated penciclovir, and 6-deoxy monoacetylated penciclovir (5%, <0.5% and <0.5% of the dose in the urine, respectively). Little or no famciclovir is detected in plasma or urine. An in vitro study using human liver microsomes demonstrated that cytochrome P450 does not play an important role in famciclovir metabolism. The conversion of 6-deoxy penciclovir to penciclovir is catalyzed by aldehyde oxidase. Cimetidine and promethazine, in vitro inhibitors of aldehyde oxidase, did not show relevant effects on the formation of penciclovir in vivo [see Drug Interactions (7.2)].
Elimination: Approximately 94% of administered radioactivity was recovered in urine over 24 hours (83% of the dose was excreted in the first 6 hours) after the administration of 5 mg/kg radiolabeled penciclovir as a 1-hour infusion to three healthy male volunteers. Penciclovir accounted for 91% of the radioactivity excreted in the urine.
Following the oral administration of a single 500 mg dose of radiolabeled famciclovir to three healthy male volunteers, 73% and 27% of administered radioactivity were recovered in urine and feces over 72 hours, respectively. Penciclovir accounted for 82% and 6-deoxy penciclovir accounted for 7% of the radioactivity excreted in the urine. Approximately 60% of the administered radiolabeled dose was collected in urine in the first 6 hours.
After intravenous administration of penciclovir in 48 healthy male volunteers, mean ± S.D. total plasma clearance of penciclovir was 36.6±6.3 L/hr (0.48±0.09 L/hr/kg). Penciclovir renal clearance accounted for 74.5±8.8% of total plasma clearance.
Renal clearance of penciclovir following the oral administration of a single 500 mg dose of famciclovir to 109 healthy male volunteers was 27.7±7.6 L/hr. Active tubular secretion contributes to the renal elimination of penciclovir.
The plasma elimination half-life of penciclovir was 2.0±0.3 hours after intravenous administration of penciclovir to 48 healthy male volunteers and 2.3±0.4 hours after oral administration of 500-mg famciclovir to 124 healthy male volunteers. The half-life in 17 patients with herpes zoster was 2.8±1.0 hours and 2.7±1.0 hours after single and repeated doses, respectively.
Special populations:
Geriatric patients: Based on cross study comparison, penciclovir AUC was 40% higher and penciclovir renal clearance was 22% lower in elderly subjects (n=18, age 65 to 79 years) as compared with younger subjects Some of this difference may be due to differences in renal function between the two groups. No famciclovir dosage adjustment based on age is recommended unless renal function is impaired [see Dosage and Administration (2.3), Use in Specific Populations (8.5)].
Patients with renal impairment: In subjects with varying degrees of renal impairment, apparent plasma clearance, renal clearance, and the plasma-elimination rate constant of penciclovir decreased linearly with reductions in renal function, both after single and repeated dosing [see Use Specific Populations (8.6)]. A dosage adjustment is recommended for patients with renal impairment [see Dosage and Administration (2.3)].
Patients with hepatic impairment: Well-compensated chronic liver disease had no effect on the extent of availability (AUC) of penciclovir [see Use in Specific Populations (8.7)]. No dosage adjustment is recommended for patients with well-compensated hepatic impairment.
HIV-infected patients: Following oral administration of a single dose of 500-mg famciclovir to HIV-positive patients, the pharmacokinetic parameters of penciclovir were comparable to those observed in healthy subjects.
Gender: The pharmacokinetics of penciclovir were evaluated in 18 healthy male and 18 healthy female volunteers after single-dose oral administration of 500-mg famciclovir. AUC of penciclovir was 9.3±1.9 mcg hr/mL and 11.1±2.1 mcg hr/mL in males and females, respectively. Penciclovir renal clearance was 28.5±8.9 L/hr and 21.8±4.3 L/hr, respectively. These differences were attributed to differences in renal function between the two groups. No famciclovir dosage adjustment based on gender is recommended.
Race: A retrospective evaluation was performed to compare the pharmacokinetic parameters obtained in Black and Caucasian subjects after single and repeat once-daily, twice-daily, or three times-daily administration of famciclovir 500 mg. Data from a study in healthy volunteers (single dose), a study in subjects with varying degrees of renal impairment (single and repeat dose) and a study in subjects with hepatic impairment (single dose) did not indicate any significant differences in the pharmacokinetics of penciclovir between Black and Caucasian subjects.
12.4 Virology
Mechanism of action: Famciclovir is a prodrug of penciclovir, which has demonstrated inhibitory activity against herpes simplex virus types 1 (HSV-1) and 2 (HSV-2) and varicella zoster virus (VZV). In cells infected with HSV-1, HSV-2 or VZV, the viral thymidine kinase phosphorylates penciclovir to a monophosphate form that, in turn, is converted by cellular kinases to the active form penciclovir triphosphate. Biochemical studies demonstrate that penciclovir triphosphate inhibits HSV-2 DNA polymerase competitively with deoxyguanosine triphosphate. Consequently, herpes viral DNA synthesis and, therefore, replication are selectively inhibited. Penciclovir triphosphate has an intracellular half-life of 10 hours in HSV-1-, 20 hours in HSV-2- and 7 hours in VZV-infected cells grown in culture; however, the clinical significance of the intracellular half-life is unknown.
Antiviral activity: In cell culture studies, penciclovir is inhibitory to the following herpes viruses: HSV-1, HSV-2 and VZV. The antiviral activity of penciclovir against wild type strains grown on human foreskin fibroblasts was assessed with a plaque reduction assay and staining with crystal violet 3 days postinfection for HSV and 10 days postinfection for VZV. The median EC50 values of penciclovir against laboratory and clinical isolates of HSV-1, HSV-2, and VZV were 2 µM (range 1.2 to 2.4 µM, n = 7), 2.6 µM (range 1.6 to 11 µM, n = 6), and 34 µM (range 6.7 to 71 µM, n = 6), respectively.
Resistance: Penciclovir-resistant mutants of HSV and VZV can result from mutations in the viral thymidine kinase (TK) and DNA polymerase genes. Mutations in the viral TK gene may lead to complete loss of TK activity (TK negative), reduced levels of TK activity (TK partial), or alteration in the ability of viral TK to phosphorylate the drug without an equivalent loss in the ability to phosphorylate thymidine (TK altered). The most commonly encountered acyclovir resistant mutants that are TK negative are also resistant to penciclovir. The median EC50 values observed in a plaque reduction assay with penciclovir resistant HSV-1, HSV-2, and VZV were 69 µM (range 14 to 115 µM, n = 6), 46 µM (range 4 to >395 µM, n = 9), and 92 µM (range 51 to 148 µM, n = 4), respectively. The possibility of viral resistance to penciclovir should be considered in patients who fail to respond or experience recurrent viral shedding during therapy.
Cross-resistance: Cross-resistance has been observed among HSV DNA polymerase inhibitors. The most commonly encountered acyclovir resistant mutants that are TK negative are also resistant to penciclovir.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
Carcinogenesis: Two-year dietary carcinogenicity studies with famciclovir were conducted in rats and mice. An increase in the incidence of mammary adenocarcinoma (a common tumor in animals of this strain) was seen in female rats receiving the high dose of 600 mg/kg/day (1.1 to 4.5x the human systemic exposure at the recommended total daily oral dose ranging between 500 mg and 2000 mg, based on area under the plasma concentration curve comparisons [24 hr
AUC] for penciclovir). No increases in tumor incidence were reported in male rats treated at doses up to 240 mg/kg/day (0.7 to 2.7x the human AUC), or in male and female mice at doses up to 600 mg/kg/day (0.3 to 1.2x the human AUC).
Mutagenesis: Famciclovir and penciclovir (the active metabolite of famciclovir) were tested for genotoxic potential in a battery of in vitro and in vivo assays. Famciclovir and penciclovir were negative in in vitro tests for gene mutations in bacteria (S. typhimurium and E. coli) and unscheduled DNA synthesis in mammalian HeLa 83 cells (at doses up to 10,000 and 5,000 mcg/plate, respectively). Famciclovir was also negative in the L5178Y mouse lymphoma assay (5000 mcg/mL), the in vivo mouse micronucleus test (4800 mg/kg), and rat dominant lethal study (5000 mg/kg). Famciclovir induced increases in polyploidy in human lymphocytes in vitro in the absence of chromosomal damage (1200 mcg/mL). Penciclovir was positive in the L5178Y mouse lymphoma assay for gene mutation/chromosomal aberrations, with and without metabolic activation (1000 mcg/mL). In human lymphocytes, penciclovir caused chromosomal aberrations in the absence of metabolic activation (250 mcg/mL). Penciclovir caused an increased incidence of micronuclei in mouse bone marrow in vivo when administered intravenously at doses highly toxic to bone marrow (500 mg/kg), but not when administered orally.
Impairment of fertility: Testicular toxicity was observed in rats, mice, and dogs following repeated administration of famciclovir or penciclovir. Testicular changes included atrophy of the seminiferous tubules, reduction in sperm count, and/or increased incidence of sperm with abnormal morphology or reduced motility. The degree of toxicity to male reproduction was related to dose and duration of exposure. In male rats, decreased fertility was observed after 10 weeks of dosing at 500 mg/kg/day (1.4 to 5.7x the human AUC). The no observable effect level for sperm and testicular toxicity in rats following chronic administration (26 weeks) was 50 mg/kg/day (0.15 to 0.6x the human systemic exposure based on AUC comparisons). Testicular toxicity was observed following chronic administration to mice (104 weeks) and dogs (26 weeks) at doses of 600 mg/kg/day (0.3 to 1.2x the human AUC) and 150 mg/kg/day (1.3 to 5.1x the human AUC), respectively.
Famciclovir had no effect on general reproductive performance or fertility in female rats at doses up to 1000 mg/kg/day (2.7 to 10.8x the human AUC).
Two placebo-controlled studies in a total of 130 otherwise healthy men with a normal sperm profile over an 8 week baseline period and recurrent genital herpes receiving oral famciclovir (250 mg twice daily) (n=66) or placebo (n=64) therapy for 18 weeks showed no evidence of significant effects on sperm count, motility or morphology during treatment or during an 8-week follow-up.
13.2 Animal Pharmacology & OR Toxicology
Juvenile toxicity study in rats: In juvenile rats, famciclovir was administered daily at doses of 0, 40, 125, or 400 mg/kg/day for 10 weeks beginning on post-partum Day 4. There were no treatment related deaths or clinical observations. The toxicity of famciclovir was not enhanced in juvenile rats compared to that in the adult animals.
14 CLINICAL STUDIES
14.1 Herpes Labialis (Cold Sores)
A randomized, double-blind, placebo-controlled trial was conducted in 701 immunocompetent adults with recurrent herpes labialis. Patients self-initiated therapy within 1 hour of first onset of signs or symptoms of a recurrent herpes labialis episode with famciclovir1500 mg as a single dose (n=227), famciclovir 750 mg twice daily (n=220) or placebo (n=254) for 1 day. The median time to healing among patients with non-aborted lesions (progressing beyond the papule stage) was 4.4 days in the famciclovir 1500 mg single-dose group (n=152) as compared to 6.2 days in the placebo group (n=168). The median difference in time to healing between the placebo and famciclovir 1500 mg treated groups was 1.3 days (95% CI: 0.6 – 2.0). No differences in proportion of patients with aborted lesions (not progressing beyond the papule stage) were observed between patients receiving famciclovir or placebo: 33% for famciclovir 1500 mg single dose and 34% for placebo. The median time to loss of pain and tenderness was 1.7 days in famciclovir 1500 mg single dose-treated patients versus 2.9 days in placebo-treated patients.
14.2 Genital Herpes
Recurrent episodes: A randomized, double-blind, placebo-controlled trial was conducted in 329 immunocompetent adults with recurrent genital herpes. Patients self-initiated therapy within 6 hours of the first sign or symptom of a recurrent genital herpes episode with either famciclovir 1000 mg twice daily (n=163) or placebo (n=166) for 1 day. The median time to healing among patients with non-aborted lesions (progressing beyond the papule stage) was 4.3 days in famciclovir-treated patients (n=125) as compared to 6.1 days in placebo-treated patients (n=145). The median difference in time to healing between the placebo and famciclovir-treated groups was 1.2 days (95% CI: 0.5 – 2.0). Twenty-three percent of famciclovir-treated patients had aborted lesions (no lesion development beyond erythema) versus 13% in placebo-treated patients. The median time to loss of all symptoms (e.g., tingling, itching, burning, pain, or tenderness) was 3.3 days in famciclovir-treated patients vs. 5.4 days in placebo-treated patients.
Suppressive therapy: Two randomized, double-blind, placebo-controlled, 12-month trials were conducted in 934 immunocompetent adults with a history of 6 or more recurrences of genital herpes episodes per year. Comparisons included famciclovir 125 mg three times daily, 250 mg twice daily, 250 mg three times daily, and placebo. At 12 months, 60% to 65% of patients were still receiving famciclovir and 25% were receiving placebo treatment. Recurrence rates at 6 and 12 months in patients treated with the 250 mg twice daily dose are shown in Table 6.
| Recurrence Rates at 6 Months |
Recurrence Rates at 12 Months |
|||
| Famciclovir 250 mg b.i.d. (n=236) |
Placebo (n=233) |
Famciclovir 250 mg b.i.d. (n=236) |
Placebo (n=233) |
|
| Recurrence-free | 39% | 10% | 29% | 6% |
Recurrences |
47% | 74% | 53% | 78% |
Lost to Follow-up |
14% | 16% | 17% | 16% |
Famciclovir -treated patients had approximately 1/5 the median number of recurrences as compared to placebo-treated patients. Higher doses of famciclovir were not associated with an increase in efficacy.
14.3 Recurrent Orolabial or Genital Herpes in HIV-Infected Patients
A randomized, double-blind trial compared famciclovir 500 mg twice daily for 7 days (n=150) with oral acyclovir 400 mg 5 times daily for 7 days (n=143) in HIV-infected patients with recurrent orolabial or genital herpes treated within 48 hours of lesion onset. Approximately 40% of patients had a CD4+ count below 200 cells/mm3, 54% of patients had anogenital lesions and 35% had orolabial lesions. Famciclovir therapy was comparable to oral acyclovir in reducing new lesion formation and in time to complete healing.
14.4 Herpes Zoster (Shingles)
Two randomized, double-blind trials, 1 placebo-controlled and 1 active-controlled, were conducted in 964 immunocompetent adults with uncomplicated herpes zoster. Treatment was initiated within 72 hours of first lesion appearance and was continued for 7 days.
In the placebo-controlled trial, 419 patients were treated with either famciclovir 500 mg three times daily (n=138), famciclovir 750 mg three times daily (n=135) or placebo (n=146). The median time to full crusting was 5 days among famciclovir 500 mg-treated patients as compared to 7 days in placebo-treated patients. The times to full crusting, loss of vesicles, loss of ulcers, and loss of crusts were shorter for famciclovir 500 mg-treated patients than for placebo-treated patients in the overall study population. The effects of famciclovir were greater when therapy was initiated within 48 hours of rash onset; it was also more profound in patients 50 years of age or older. Among the 65.2% of patients with at least 1 positive viral culture, famciclovir treated patients had a shorter median duration of viral shedding than placebo-treated patients (1 day and 2 days, respectively).
There were no overall differences in the duration of pain before rash healing between famciclovir- and placebo-treated groups. In addition, there was no difference in the incidence of pain after rash healing (postherpetic neuralgia) between the treatment groups. In the 186 patients (44.4% of total study population) who developed postherpetic neuralgia, the median duration of postherpetic neuralgia was shorter in patients treated with famciclovir 500 mg than in those treated with placebo (63 days and 119 days, respectively). No additional efficacy was demonstrated with higher dose of famciclovir.
In the active-controlled trial, 545 patients were treated with one of three doses of famciclovir three times daily or with acyclovir 800 mg five times daily. Times to full lesion crusting and times to loss of acute pain were comparable for all groups and there were no statistically significant differences in the time to loss of postherpetic neuralgia between famciclovir and acyclovir-treated groups.
16 HOW SUPPLIED/STORAGE AND HANDLING
Dispensed in blister punch material for Institutional Use Only.Famciclovir tablets are supplied as film-coated tablets as follows:
• Famciclovir 125 mg tablet: White to off white, round film-coated, biconvex, engraved with “ML 67” on one side and plain on the other side.
125 mg ……NDC 50268-305-13 10 Tablets per card, 3 cards per carton
• Famciclovir 250 mg tablet: White to off white, round film-coated, biconvex, engraved with “ML 70” on one side and plain on the other side.
250 mg ……NDC 50268-306-13 10 Tablets per card, 3 cards per carton
• Famciclovir 500 mg tablet: White to off white, oval film-coated, biconvex, engraved with “ML 72” on one side and plain on the other side.
500 mg ……NDC 50268-307-13 10 Tablets per card, 3 cards per carton
Dispensed in blister punch material for Institutional Use Only.
Store at 20° - 25°C (68°-77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (Patient Information)
There is no evidence that famciclovir will affect the ability of a patient to drive or to use machines. However, patients who experience dizziness, somnolence, confusion or other central nervous system disturbances while taking famciclovir tablets should refrain from driving or operating machinery.
Because famciclovir tablet contains lactose (Famciclovir 125 mg, 250 mg and 500 mg tablets contain lactose 25.97 mg, 51.95 mg and 103.90 mg, respectively), patients with rare hereditary problems of galactose intolerance, a severe lactase deficiency or glucose-galactose malabsorption should be advised to discuss with their healthcare provider before taking famciclovir tablets.
17.1 Herpes Labialis (Cold Sores)
Patients should be advised to initiate treatment at the earliest sign or symptom of a recurrence of cold sores (e.g., tingling, itching, burning, pain, or lesion). Patients should be instructed that treatment for cold sores should not exceed 1 dose. Patients should be informed that famciclovir tablets are not a cure for cold sores.
17.2 Genital Herpes
Patients should be informed that famciclovir tablets are not a cure for genital herpes. There are no data evaluating whether famciclovir tablets will prevent transmission of infection to others. Because genital herpes is a sexually transmitted disease, patients should avoid contact with lesions or intercourse when lesions and/or symptoms are present to avoid infecting partners. Genital herpes is frequently transmitted in the absence of symptoms through asymptomatic viral shedding. Therefore, patients should be counseled to use safer sex practices.
If episodic therapy for recurrent genital herpes is indicated, patients should be advised to initiate therapy at the first sign or symptom of an episode.
There are no data on safety or effectiveness of chronic suppressive therapy of longer than one year duration.
17.3 Herpes Zoster (Shingles)
There are no data on treatment initiated more than 72 hours after onset of zoster rash. Patients should be advised to initiate treatment as soon as possible after a diagnosis of herpes zoster.
Denavir® is a registered trademark of Novartis Consumer Health, Inc.
Patient Information
Famciclovir Tablets
Read this Patient Information before you start taking famciclovir tablets and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
What is Famciclovir Tablet?
Famciclovir tablet is a prescription antiviral medicine used to:
• treat outbreaks of cold sores (fever blisters) in healthy adults
• treat outbreaks of genital herpes in healthy adults
• decrease the number of outbreaks of genital herpes in healthy adults
• treat outbreaks of herpes simplex lesions in or around the mouth, genitals, and anal area in people infected with HIV
• treat shingles (herpes zoster) in adults with normal immune system
It is not known if famciclovir tablets are safe and effective in children younger than 18 years of age.
Famciclovir tablets are not a cure for herpes. It is not known if famciclovir tablets can stop the spread of herpes to others. If you are sexually active, you can pass herpes to your partner even if you are taking famciclovir tablets. Herpes can be transmitted even if you do not have active symptoms. You should continue to practice safer sex to lower the chances of spreading genital herpes to others. Do not have sexual contact with your partner during an outbreak of genital herpes or if you have any symptoms of genital herpes. Use a condom made of latex or polyurethane when you have a sexual contact. Ask your healthcare provider for more information about safer sex practices.
Who should not take Famciclovir Tablets?
Do not take famciclovir tablets if you are allergic to any of its ingredients or to Denavir®(penciclovir cream). See the end of this Patient Information leaflet for a complete list of ingredients in famciclovir tablets.
What should I tell my healthcare provider before taking Famciclovir tablets?
Before you start taking famciclovir tablets, tell your healthcare provider if you:
• have kidney or liver problems
• have a rare genetic problem with galactose intolerance, a severe lactase deficiency or you do not absorb glucose-galactose (malabsorption)
• are pregnant or planning to become pregnant. It is not known if famciclovir tablets will harm your unborn baby
• are breast-feeding or plan to breast-feed.
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take:
• any other medicines and products you use to treat herpes outbreaks
• probenecid (Probalan®)
Know the medicines you take. Keep a list of them with you to show to your healthcare provider and pharmacist every time you get a new medicine.
How should I take Famciclovir Tablets?
• Take famciclovir tablets exactly as prescribed
• Your healthcare provider will tell you how many famciclovir tablets to take and when to take them. Your dose of famciclovir tablets and how often you take it may be different depending on your condition
• Famciclovir tablets can be taken with or without food
• It is important for you to finish all of the medicine as prescribed, even if you begin to feel better
• Your symptoms may continue even after you finish all of your famciclovir tablets. This does not mean that you need more medicine, since you have already finished a full course of famciclovir tablets and it will continue to work in your body. Talk to your healthcare provider if you have any questions about your condition and your treatment.
What are the possible side effects of Famciclovir Tablets?
The most common side effects of Famciclovir tablets include:
• headache
• nausea
Talk to your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of famciclovir tablets. Ask your healthcare provider or pharmacist for more information.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Famciclovir Tablets?
• Store famciclovir tablets at room temperature between 59° and 86°F (15° to 30°C).
Keep Famciclovir tablets and all medicines out of reach from children.
General information about Famciclovir Tablets
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use famciclovir tablets for a condition for which it was not prescribed. Do not give famciclovir tablets to other people, even if they have the same symptoms you have. It may harm them. This leaflet summarizes the most important information about famciclovir tablets. If you would like more information, talk with your healthcare provider. Your healthcare provider or pharmacist can give you information about famciclovir tablets that is written for health professionals.
For more information, call 1-888-943-3210.
What are the ingredients in Famciclovir Tablets?
Active ingredient: famciclovir
Inactive ingredients: hydroxypropyl cellulose, hypromellose , anhydrous lactose, magnesium stearate, polyethylene glycols, sodium starch glycolate, and titanium dioxide.
Denavir® is a registered trademark of Novartis Consumer Healh, Inc.
Probalan® is a registered trademark of Lannet company Inc.
Manufactured for:
AvKARE, Inc.
Pulaski, TN 38478
Mfg. Rev. 07/12
AvPAK
AV 05/13 (P)
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 50268-305-13
Famciclovir Tablets
125 mg
Rx Only
30 Tablets (3 X 10) Unit Dose
5026830513
NDC 50268-305-13
Famciclovir Tablets
125 mg
Rx Only
30 Tablets (3 X 10) Unit Dose
5026830513
Pharmacist: Dispense the Patient Information Leaflet with The Drug Product.
Each film coated tablet contains:
Famciclovir 125 mg
USUAL DOSAGE: See Prescribing Information
Store at 20oC to 25oC (68oF to 77oF) [See USP controlled room temperature].
Manufactured for:
AvKARE, Inc.
Pulaski, TN 38478
AvPAK
A PRODUCT OF AvKARE
Mfg. PM01265801 AV 05/13 (P)
NDC 50268-306-13
Famciclovir Tablets
250 mg
Rx Only
30 Tablets (3 X 10) Unit Dose
5026830613
NDC 50268-306-13
Famciclovir Tablets
250 mg
Rx Only
30 Tablets (3 X 10) Unit Dose
5026830613
Pharmacist: Dispense the Patient Information Leaflet with The Drug Product.
Each film coated tablet contains:
Famciclovir 250 mg
USUAL DOSAGE: See Prescribing Information
Store at 20oC to 25oC (68oF to 77oF) [See USP controlled room temperature].
Manufactured for:
AvKARE, Inc.
Pulaski, TN 38478
AvPAK
A PRODUCT OF AvKARE
Mfg. PM01265901 AV 05/13 (P)
NDC 50268-307-13
Famciclovir Tablets
500 mg
Rx Only
30 Tablets (3 X 10) Unit Dose
5026830713
NDC 50268-307-13
Famciclovir Tablets
500 mg
Rx Only
30 Tablets (3 X 10) Unit Dose
5026830713
Pharmacist: Dispense the Patient Information Leaflet with The Drug Product.
Each film coated tablet contains:
Famciclovir 500 mg
USUAL DOSAGE: See Prescribing Information
Store at 20oC to 25oC (68oF to 77oF) [See USP controlled room temperature].
Manufactured for:
AvKARE, Inc.
Pulaski, TN 38478
AvPAK
A PRODUCT OF AvKARE
Mfg. PM01266001 AV 05/13 (P)
FamciclovirFamciclovir TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
FamciclovirFamciclovir TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
FamciclovirFamciclovir TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||