Fenofibrate
FULL PRESCRIBING INFORMATION: CONTENTS*
- FENOFIBRATE DESCRIPTION
- INACTIVE INGREDIENT
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS & USAGE
- FENOFIBRATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- FENOFIBRATE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
FENOFIBRATE DESCRIPTION
DESCRIPTION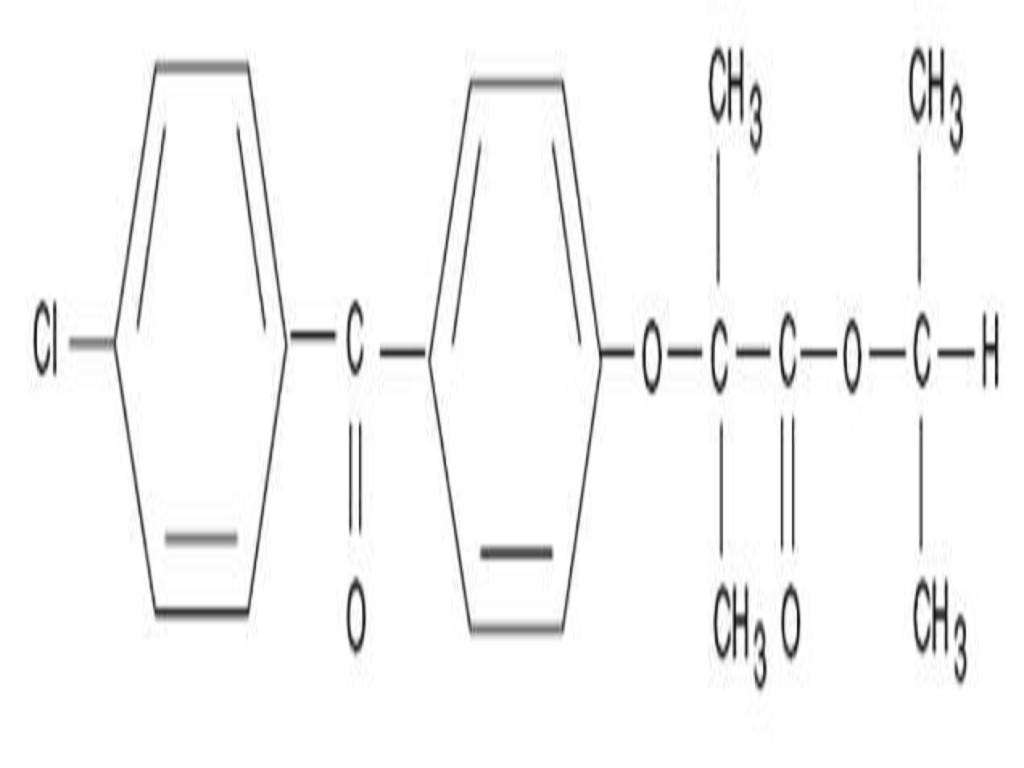
INACTIVE INGREDIENT
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGYPHARMACOKINETICS
Pharmacokinetics/MetabolismAbsorption
Distribution
Metabolism
Excretion
Special Populations
Geriatrics
Pediatrics
Gender
Race
Renal insufficiency
Hepatic insufficiency
Drug-drug interactions
WARNINGSPRECAUTIONS
Clinical Trials
Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia (Fredrickson Types IIa and IIb)
Table 1
*
Treatment GroupTotal-CLDL-CHDL-CTG*
Hypertriglyceridemia (Fredrickson Type IV and V)
Table 2
Study 1PlaceboFenofibrate TabletsBaseline TG levels 350 to NBaseEndpoint% ChangeNBaseEndpoint% Change499 mg/dLline(Mean)(Mean)line(Mean)(Mean)MeanMean*****Study 2PlaceboFenofibrate Tablets*******
INDICATIONS & USAGE
INDICATIONS AND USAGETreatment of Hypercholesterolemia
National Cholesterol Education Program [NCEP] Treatment Guidelines
Treatment of Hypertriglyceridemia
WARNINGSPRECAUTIONS
TypeLipoprotein ElevatedLipid ElevationMajorMinor
DefiniteTwo or More OtherLDL-Cholesterol mg/dL (mmol/L)AthleroscleroticRisk Factors Initiation LevelGoalDisease* *
FENOFIBRATE CONTRAINDICATIONS
WARNINGS
WARNINGS
WARNINGSLiver Function
Cholelithiasis
Concomitant Oral Anticoagulants
Concomitant HMG-CoA reductase inhibitors
Mortality
Other Considerations
PRECAUTIONS
PRECAUTIONSInitial Therapy
Continued therapy
Pancreatitis
Hypersensitivity Reactions
Hematologic Changes
Skeletal muscle
DRUG INTERACTIONS
Drug InteractionsOral Anticoagulants
HMG-CoA reductase inhibitors
WARNINGS
Resins
Cyclosporine
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis, Impairment of FertilityPREGNANCY
Pregnancy Category CNURSING MOTHERS
Nursing mothersPEDIATRIC USE
Pediatric UseGERIATRIC USE
Geriatric UseFENOFIBRATE ADVERSE REACTIONS
CLINICALBODY SYSTEM Adverse EventFenofibrate*PLACEBO(N=439)(N=365)BODY AS A WHOLEDIGESTIVEMETABOLIC AND NUTRITIONAL DISORDERSRESPIRATORY*
OVERDOSAGE
OVERDOSAGEDOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATIONHOW SUPPLIED
STORAGE AND HANDLING
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

FenofibrateFenofibrate CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!