Fentanyl Citrate
West-ward Pharmaceutical Corp.
FULL PRESCRIBING INFORMATION: CONTENTS*
- FENTANYL CITRATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- FENTANYL CITRATE INDICATIONS AND USAGE
- FENTANYL CITRATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- FENTANYL CITRATE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- FENTANYL CITRATE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
CII
Rx only
FENTANYL CITRATE DESCRIPTION
Fentanyl Citrate Injection is a sterile, non-pyrogenic solution for intravenous or intramuscular use as a potent narcotic analgesic. Each mL contains fentanyl citrate equivalent to 50 mcg (0.05 mg) fentanyl base in Water for Injection. pH 4.0–7.5; sodium hydroxide and/or hydrochloric acid added, if needed, for pH adjustment. Contains no preservative.
Fentanyl citrate is chemically identified as N‑(1‑Phenethyl‑4‑piperidyl)propionanilide citrate (1:1) with the following structural formula:

CLINICAL PHARMACOLOGY
Fentanyl citrate is a narcotic analgesic. A dose of 100 mcg (0.1 mg) (2 mL) is approximately equivalent in analgesic activity to 10 mg of morphine or 75 mg of meperidine. The principal actions of therapeutic value are analgesia and sedation. Alterations in respiratory rate and alveolar ventilation, associated with narcotic analgesics, may last longer than the analgesic effect. As the dose of narcotic is increased, the decrease in pulmonary exchange becomes greater. Large doses may produce apnea. Fentanyl appears to have less emetic activity than either morphine or meperidine. Histamine assays and skin wheal testing in man indicate that clinically significant histamine release rarely occurs with fentanyl. Recent assays in man show no clinically significant histamine release in dosages up to 50 mcg/kg (0.05 mg/kg) (1 mL/kg). Fentanyl preserves cardiac stability and blunts stress-related hormonal changes at higher doses.
The pharmacokinetics of fentanyl can be described as a three-compartment model, with a distribution time of 1.7 minutes, redistribution of 13 minutes and a terminal elimination half-life of 219 minutes. The volume of distribution for fentanyl is 4 L/kg.
Fentanyl plasma protein binding capacity increases with increasing ionization of the drug. Alterations in pH may affect its distribution between plasma and the central nervous system. It accumulates in skeletal muscle and fat and is released slowly into the blood. Fentanyl, which is primarily transformed in the liver, demonstrates a high first pass clearance and releases approximately 75% of an intravenous dose in urine, mostly as metabolites with less than 10% representing the unchanged drug. Approximately 9% of the dose is recovered in the feces, primarily as metabolites.
The onset of action of fentanyl is almost immediate when the drug is given intravenously; however, the maximal analgesic and respiratory depressant effect may not be noted for several minutes. The usual duration of action of the analgesic effect is 30 to 60 minutes after a single intravenous dose of up to 100 mcg (0.1 mg) (2 mL). Following intramuscular administration, the onset of action is from seven to eight minutes, and the duration of action is one to two hours. As with longer acting narcotic analgesics, the duration of the respiratory depressant effect of fentanyl may be longer than the analgesic effect. The following observations have been reported concerning altered respiratory response to CO2 stimulation following administration of fentanyl citrate to man:
- DIMINISHED SENSITIVITY TO CO2 STIMULATION MAY PERSIST LONGER THAN DEPRESSION OF RESPIRATORY RATE. (Altered sensitivity to CO2 stimulation has been demonstrated for up to four hours following a single dose of 600 mcg [0.6 mg] [12 mL] fentanyl to healthy volunteers.) Fentanyl frequently slows the respiratory rate, duration and degree of respiratory depression being dose related.
- The peak respiratory depressant effect of a single intravenous dose of fentanyl citrate is noted 5 to 15 minutes following injection. See also WARNINGS and PRECAUTIONS concerning respiratory depression.
FENTANYL CITRATE INDICATIONS AND USAGE
Fentanyl Citrate Injection is indicated:
- for analgesic action of short duration during the anesthetic periods, premedication, induction and maintenance, and in the immediate postoperative period (recovery room) as the need arises.
- for use as a narcotic analgesic supplement in general or regional anesthesia.
- for administration with a neuroleptic such as droperidol injection as an anesthetic premedication, for the induction of anesthesia and as an adjunct in the maintenance of general and regional anesthesia.
- for use as an anesthetic agent with oxygen in selected high risk patients, such as those undergoing open heart surgery or certain complicated neurological or orthopedic procedures.
FENTANYL CITRATE CONTRAINDICATIONS
Fentanyl Citrate Injection is contraindicated in patients with known intolerance to the drug.
WARNINGS
FENTANYL CITRATE SHOULD BE ADMINISTERED ONLY BY PERSONS SPECIFICALLY TRAINED IN THE USE OF INTRAVENOUS ANESTHETICS AND MANAGEMENT OF THE RESPIRATORY EFFECTS OF POTENT OPIOIDS.
AN OPIOID ANTAGONIST, RESUSCITATIVE AND INTUBATION EQUIPMENT AND OXYGEN SHOULD BE READILY AVAILABLE.
See also discussion of narcotic antagonists in PRECAUTIONS and OVERDOSAGE .
If fentanyl is administered with a tranquilizer such as droperidol, the user should become familiar with the special properties of each drug, particularly the widely differing durations of action. In addition, when such a combination is used, fluids and other countermeasures to manage hypotension should be available.
As with other potent narcotics, the respiratory depressant effect of fentanyl may persist longer than the measured analgesic effect. The total dose of all narcotic analgesics administered should be considered by the practitioner before ordering narcotic analgesics during recovery from anesthesia. It is recommended that narcotics, when required, should be used in reduced doses initially, as low as 1/4 to 1/3 those usually recommended.
Fentanyl may cause muscle rigidity, particularly involving the muscles of respiration. In addition, skeletal muscle movements of various groups in the extremities, neck and external eye have been reported during induction of anesthesia with fentanyl; these reported movements have, on rare occasions, been strong enough to pose patient management problems. This effect is related to the dose and speed of injection and its incidence can be reduced by: 1) administration of up to 1/4 of the full paralyzing dose of a non-depolarizing neuromuscular blocking agent just prior to administration of fentanyl citrate; 2) administration of a full paralyzing dose of a neuromuscular blocking agent following loss of eyelash reflex when fentanyl is used in anesthetic doses titrated by slow intravenous infusion; or, 3) simultaneous administration of fentanyl citrate and a full paralyzing dose of neuromuscular blocking agent when fentanyl citrate is used in rapidly administered anesthetic dosages. The neuromuscular blocking agent used should be compatible with the patient’s cardiovascular status.
Adequate facilities should be available for postoperative monitoring and ventilation of patients administered anesthetic doses of fentanyl. Where moderate or high doses are used (above 10 mcg/kg), there must be adequate facilities for postoperative observation, and ventilation if necessary, of patients who have received fentanyl. It is essential that these facilities be fully equipped to handle all degrees of respiratory depression.
Fentanyl may also produce other signs and symptoms characteristic of narcotic analgesics including euphoria, miosis, bradycardia and bronchoconstriction.
Severe and unpredictable potentiation by MAO inhibitors has been reported for other narcotic analgesics. Although this has not been reported for fentanyl, there are insufficient data to establish that this does not occur with fentanyl. Therefore, when fentanyl is administered to patients who have received MAO inhibitors within 14 days, appropriate monitoring and ready availability of vasodilators and beta-blockers for the treatment of hypertension is indicated.
Head Injuries and Increased Intracranial Pressure
Fentanyl should be used with caution in patients who may be particularly susceptible to respiratory depression, such as comatose patients who may have a head injury or brain tumor. In addition, fentanyl may obscure the clinical course of patients with head injury.
PRECAUTIONS
General
The initial dose of fentanyl citrate should be appropriately reduced in elderly and debilitated patients. The effect of the initial dose should be considered in determining incremental doses.
Nitrous oxide has been reported to produce cardiovascular depression when given with higher doses of fentanyl.
Certain forms of conduction anesthesia, such as spinal anesthesia and some peridural anesthetics, can alter respiration by blocking intercostal nerves. Through other mechanisms (See CLINICAL PHARMACOLOGY ) fentanyl can also alter respiration. Therefore, when fentanyl is used to supplement these forms of anesthesia, the anesthetist should be familiar with the physiological alterations involved, and be prepared to manage them in the patients selected for these forms of anesthesia.
When a tranquilizer such as droperidol is used with fentanyl, pulmonary arterial pressure may be decreased. This fact should be considered by those who conduct diagnostic and surgical procedures where interpretation of pulmonary arterial pressure measurements might determine final management of the patient. When high dose or anesthetic dosages of fentanyl are employed, even relatively small dosages of diazepam may cause cardiovascular depression. When fentanyl is used with a tranquilizer such as droperidol, hypotension can occur. If it occurs, the possibility of hypovolemia should also be considered and managed with appropriate parenteral fluid therapy. Repositioning the patient to improve venous return to the heart should be considered when operative conditions permit. Care should be exercised in moving and positioning of patients because of the possibility of orthostatic hypotension. If volume expansion with fluids plus other countermeasures do not correct hypotension, the administration of pressor agents other than epinephrine should be considered. Because of the alpha-adrenergic blocking action of droperidol, epinephrine may paradoxically decrease the blood pressure in patients treated with droperidol.
Elevated blood pressure, with and without pre-existing hypertension, has been reported following administration of fentanyl citrate combined with droperidol. This might be due to unexplained alterations in sympathetic activity following large doses; however, it is also frequently attributed to anesthetic and surgical stimulation during light anesthesia.
When droperidol is used with fentanyl and the EEG is used for postoperative monitoring, it may be found that the EEG pattern returns to normal slowly.
Vital signs should be monitored routinely.
Respiratory depression caused by opioid analgesics can be reversed by opioid antagonists such as naloxone. Because the duration of respiratory depression produced by fentanyl may last longer than the duration of the opioid antagonist action, appropriate surveillance should be maintained. As with all potent opioids, profound analgesia is accompanied by respiratory depression and diminished sensitivity to CO2 stimulation which may persist into or recur in the postoperative period. Intraoperative hyperventilation may further alter postoperative response to CO2. Appropriate postoperative monitoring should be employed to ensure that adequate spontaneous breathing is established and maintained in the absence of stimulation prior to discharging the patient from the recovery area.
Impaired Respiration
Fentanyl should be used with caution in patients with chronic obstructive pulmonary disease, patients with decreased respiratory reserve, and others with potentially compromised respiration. In such patients, narcotics may additionally decrease respiratory drive and increase airway resistance. During anesthesia, this can be managed by assisted or controlled respiration.
Impaired Hepatic or Renal Function
Fentanyl citrate should be administered with caution to patients with liver and kidney dysfunction because of the importance of these organs in the metabolism and excretion of drugs.
Cardiovascular Effects
Fentanyl may produce bradycardia, which may be treated with atropine. Fentanyl should be used with caution in patients with cardiac bradyarrhythmias.
Drug Interactions
Other CNS depressant drugs (e.g., barbiturates, tranquilizers, narcotics and general anesthetics) will have additive or potentiating effects with fentanyl. When patients have received such drugs, the dose of fentanyl required will be less than usual. Following the administration of fentanyl citrate, the dose of other CNS depressant drugs should be reduced.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or mutagenicity studies have been conducted with fentanyl citrate. Reproduction studies in rats revealed a significant decrease in the pregnancy rate of all experimental groups. This decrease was most pronounced in the high dosed group (1.25 mg/kg—12.5X human dose) in which one of twenty animals became pregnant.
Pregnancy
TERATOGENIC EFFECTS - PREGNANCY CATEGORY C.
Fentanyl citrate has been shown to impair fertility and to have an embryocidal effect in rats when given in doses 0.3 times the upper human dose for a period of 12 days. No evidence of teratogenic effects have been observed after administration of fentanyl citrate to rats. There are no adequate and well-controlled studies in pregnant women. Fentanyl should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery
There are insufficient data to support the use of fentanyl in labor and delivery. Therefore, such use is not recommended.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when fentanyl citrate is administered to a nursing woman.
Pediatric Use
The safety and efficacy of fentanyl citrate in pediatric patients under two years of age has not been established.
Rare cases of unexplained clinically significant methemoglobinemia have been reported in premature neonates undergoing emergency anesthesia and surgery which included combined use of fentanyl, pancuronium and atropine. A direct cause and effect relationship between the combined use of these drugs and the reported cases of methemoglobinemia has not been established.
FENTANYL CITRATE ADVERSE REACTIONS
As with other narcotic analgesics, the most common serious adverse reactions reported to occur with fentanyl are respiratory depression, apnea, rigidity and bradycardia; if these remain untreated, respiratory arrest, circulatory depression or cardiac arrest could occur. Other adverse reactions that have been reported are hypertension, hypotension, dizziness, blurred vision, nausea, emesis, laryngospasm and diaphoresis.
It has been reported that secondary rebound respiratory depression may occasionally occur postoperatively. Patients should be monitored for this possibility and appropriate countermeasures taken as necessary.
When a tranquilizer such as droperidol is used with fentanyl citrate, the following adverse reactions can occur: chills and/or shivering, restlessness and postoperative hallucinatory episodes (sometimes associated with transient periods of mental depression); extrapyramidal symptoms (dystonia, akathisia and oculogyric crisis) have been observed up to 24 hours postoperatively. When they occur, extrapyramidal symptoms can usually be controlled with anti-parkinson agents. Postoperative drowsiness is also frequently reported following the use of droperidol.
DRUG ABUSE AND DEPENDENCE
Fentanyl Citrate Injection is a Schedule II controlled drug substance that can produce drug dependence of the morphine type and, therefore, has the potential for being abused.
OVERDOSAGE
Manifestations
The manifestations of fentanyl overdosage are an extension of its pharmacologic actions (see CLINICAL PHARMACOLOGY ) as with other opioid analgesics. The intravenous LD50 of fentanyl is 3 mg/kg in rats, 1 mg/kg in cats, 14 mg/kg in dogs and 0.03 mg/kg in monkeys.
Treatment
In the presence of hypoventilation or apnea, oxygen should be administered and respiration should be assisted or controlled as indicated. A patent airway must be maintained; an oropharyngeal airway or endotracheal tube might be indicated. If depressed respiration is associated with muscular rigidity, an intravenous neuromuscular blocking agent might be required to facilitate assisted or controlled respiration. The patient should be carefully observed for 24 hours; body warmth and adequate fluid intake should be maintained. If hypotension occurs and is severe or persists, the possibility of hypovolemia should be considered and managed with appropriate parenteral fluid therapy. A specific narcotic antagonist such as naloxone should be available for use as indicated to manage respiratory depression. This does not preclude the use of more immediate countermeasures. The duration of respiratory depression following overdosage of fentanyl may be longer than the duration of narcotic antagonist action. Consult the package insert of the individual narcotic antagonists for details about use.
FENTANYL CITRATE DOSAGE AND ADMINISTRATION
The 2 mL Fentanyl Citrate Injection vial includes a cautionary label that extends above the main label and highlights the drug name, fentanyl. The purpose of the extended label is to prevent selection error with other drugs, such as Sufentanil. Read the label and confirm you have selected the correct medication. Then locate the “Tear Here” point on the label, and remove this cautionary label prior to removing the flip-off cap.
Single Dose - Destroy unused contents.
50 mcg = 0.05 mg = 1 mL
Dosage should be individualized. Some of the factors to be considered in determining the dose are age, body weight, physical status, underlying pathological condition, use of other drugs, type of anesthesia to be used and the surgical procedure involved. Dosage should be reduced in elderly or debilitated patients (see PRECAUTIONS ).
Vital signs should be monitored routinely.
I. Premedication
Premedication (to be appropriately modified in the elderly, debilitated and those who have received other depressant drugs)—50 to 100 mcg (0.05 to 0.1 mg) (1 to 2 mL) may be administered intramuscularly 30 to 60 minutes prior to surgery.
II. Adjunct to General Anesthesia
See Dosage Range Charts.
III. Adjunct to Regional Anesthesia
50 to 100 mcg (0.05 to 0.1 mg) (1 to 2 mL) may be administered intramuscularly or slowly intravenously, over one to two minutes, when additional analgesia is required.
IV. Postoperatively (recovery room)
50 to 100 mcg (0.05 to 0.1 mg) (1 to 2 mL) may be administered intramuscularly for the control of pain, tachypnea and emergence delirium. The dose may be repeated in one to two hours as needed.
Usage in Children
For induction and maintenance in children 2 to 12 years of age, a reduced dose as low as 2 to 3 mcg/kg is recommended.
|
Dosage Range Chart |
| Total Dosage (expressed as fentanyl base) |
|
Low dose—2 mcg/kg (0.002 mg/kg) (0.04 mL/kg). Fentanyl in small doses is most useful for minor, but painful, surgical procedures. In addition to the analgesia during surgery, fentanyl may also provide some pain relief in the immediate postoperative period. |
|
Moderate dose—2 to 20 mcg/kg (0.002 to 0.02 mg/kg) (0.04 to 0.4 mL/kg). Where surgery becomes more major, a larger dose is required. With this dose, in addition to adequate analgesia, one would expect to see some abolition of the stress response. However, respiratory depression will be such that artificial ventilation during anesthesia is necessary, and careful observation of ventilation postoperatively is essential. |
|
High dose—20 to 50 mcg/kg (0.02 to 0.05 mg/kg) (0.4 to 1 mL/kg). During open heart surgery and certain more complicated neurosurgical and orthopedic procedures where surgery is more prolonged, and in the opinion of the anesthesiologist, the stress response to surgery would be detrimental to the well being of the patient, dosages of 20 to 50 mcg/kg (0.02 to 0.05 mg/kg) (0.4 to 1 mL/kg) of fentanyl with nitrous oxide/oxygen have been shown to attenuate the stress response as defined by increased levels of circulating growth hormone, catecholamine, ADH and prolactin. When dosages in this range have been used during surgery, postoperative ventilation and observation are essential due to extended post-operative respiratory depression. The main objective of this technique would be to produce “stress free” anesthesia. |
|
Dosage Range Chart |
| Maintenance Dose (expressed as fentanyl base) |
| Low dose—2 mcg/kg (0.002 mg/kg) (0.04 mL/kg). Additional dosages of fentanyl are infrequently needed in these minor procedures. |
| Moderate dose—2 to 20 mcg/kg (0.002 to 0.02 mg/kg) (0.04 to 0.4 mL/kg). 25 to 100 mcg (0.025 to 0.1 mg) (0.5 to 2 mL) may be administered intravenously or intramuscularly when movement and/or changes in vital signs indicate surgical stress or lightening of analgesia. |
| High dose—20 to 50 mcg/kg (0.02 to 0.05 mg/kg) (0.4 to 1 mL/kg). Maintenance dosage (ranging from 25 mcg [0.025 mg] [0.5 mL] to one half the initial loading dose) will be dictated by the changes in vital signs which indicate stress and lightening of analgesia. However, the additional dosage selected must be individualized especially if the anticipated remaining operative time is short. |
As a General Anesthetic
When attenuation of the responses to surgical stress is especially important, doses of 50 to 100 mcg/kg (0.05 to 0.1 mg/kg) (1 to 2 mL/kg) may be administered with oxygen and a muscle relaxant. This technique has been reported to provide anesthesia without the use of additional anesthetic agents. In certain cases, doses up to 150 mcg/kg (0.15 mg/kg) (3 mL/kg) may be necessary to produce this anesthetic effect. It has been used for open heart surgery and certain other major surgical procedures in patients for whom protection of the myocardium from excess oxygen demand is particularly indicated, and for certain complicated neurological and orthopedic procedures.
As noted above, it is essential that qualified personnel and adequate facilities be available for the management of respiratory depression.
See WARNINGS and PRECAUTIONS for use of fentanyl with other CNS depressants, and in patients with altered response.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HOW SUPPLIED
Fentanyl Citrate Injection, USP, equivalent to 50 mcg (0.05 mg) fentanyl base per mL, is preservative-free and available as follows:
2 mL Single Dose ampuls packaged in 10s (NDC 0641-6024-10)
2 mL Single Dose vials packaged in 25s (NDC 0641-6027-25)
5 mL Single Dose ampuls packaged in 10s (NDC 0641-6025-10)
5 mL Single Dose vials packaged in 25s (NDC 0641-6028-25)
For Intravenous Use by Hospital Personnel Specifically Trained in the Use of Narcotic Analgesics:
20 mL Single Dose ampuls packaged in 5s (NDC 0641-6026-05)
20 mL Single Dose vials packaged in 25s (NDC 0641-6029-25)
50 mL Single Dose vials packaged individually (NDC 0641-6030-01)
Storage
PROTECT FROM LIGHT.
Keep covered in carton until time of use. Store at 20˚‑25˚C (68˚‑77˚F), excursions permitted to 15˚‑30˚C (59˚‑86˚F) [see USP Controlled Room Temperature].
To report SUSPECTED ADVERSE REACTIONS, contact West-Ward Pharmaceutical Corp. at 1-877-845-0689, or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For Product Inquiry call 1-877-845-0689.
Manufactured by:

WEST-WARD
PHARMACEUTICALS
Eatontown, NJ 07724 USA
Revised October 2011
462-254-04
PRINCIPAL DISPLAY PANEL
Fentanyl Citrate Inj., USP
CII
100 mcg/2 mL
(50 mcg/mL) (0.05 mg/mL)
2 mL Single Dose Ampul
NDC 0641-6024-01

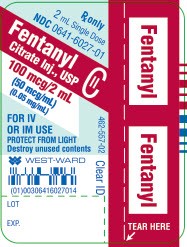
PRINCIPAL DISPLAY PANEL
Fentanyl Citrate Inj., USP
CII
100 mcg/2 mL
(50 mcg/mL)
(0.05 mg/mL)
2 mL Single Dose Vial
NDC 0641-6027-01
PRINCIPAL DISPLAY PANEL
Fentanyl Citrate Inj., USP
CII
250 mcg/5 mL
(50 mcg/mL) (0.05 mg/mL)
5 mL Single Dose Ampul
NDC 0641-6025-01

PRINCIPAL DISPLAY PANEL
Fentanyl Citrate Inj., USP
CII
250 mcg/5 mL
(50 mcg/mL) (0.05 mg/mL)
5 mL Single Dose Vial
NDC 0641-6028-01

PRINCIPAL DISPLAY PANEL
Fentanyl Citrate Injection, USP
CII
1,000 mcg/20 mL
(50 mcg/mL) (0.05 mg/mL)
20 mL Single Dose Ampul
NDC 0641-6026-01

PRINCIPAL DISPLAY PANEL
Fentanyl Citrate Injection, USP
CII
1,000 mcg/20 mL
(50 mcg/mL) (0.05 mg/mL)
20 mL Single Dose Vial
NDC 0641-6029-01

PRINCIPAL DISPLAY PANEL
Fentanyl Citrate Injection, USP
CII
2,500 mcg/50 mL
(50 mcg/mL) (0.05 mg/mL)
50 mL Single Dose Vial
NDC 0641-6030-01

Fentanyl CitrateFentanyl Citrate INJECTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fentanyl CitrateFentanyl Citrate INJECTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fentanyl CitrateFentanyl Citrate INJECTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fentanyl CitrateFentanyl Citrate INJECTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fentanyl CitrateFentanyl Citrate INJECTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fentanyl CitrateFentanyl Citrate INJECTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fentanyl CitrateFentanyl Citrate INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||