Finasteride
Bryant Ranch Prepack
Bryant Ranch Prepack
Finasteride Tablets, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- FINASTERIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- FINASTERIDE INDICATIONS AND USAGE
- FINASTERIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- FINASTERIDE ADVERSE REACTIONS
- OVERDOSAGE
- FINASTERIDE DOSAGE AND ADMINISTRATION
- Finasteride Tablets, USP
- Finasteride 5mg Tablet
FULL PRESCRIBING INFORMATION
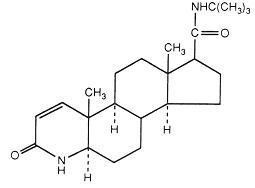
FINASTERIDE DESCRIPTION
N233622
CLINICAL PHARMACOLOGY
½in vivoin vitro
Pharmacokinetics
Drug Interactions
PRECAUTIONS, Drug Interactions
| *Range
|
|
| Mean (SD) Pharmacokinetic Parameters in Healthy Young Subjects (n=15)
|
|
|
|
Mean (± SD)
|
| Bioavailability
|
63% (34-108%)*
|
| Clearance (mL/min)
|
165 (55)
|
| Volume of Distribution (L)
|
76 (14)
|
| Half-Life (hours)
|
6.2 (2.1)
|
| *First-dose values; all other parameters are last-dose values
|
||
| Mean (SD) Noncompartmental Pharmacokinetic Parameters After Multiple Doses of 5 mg/day in Older Men
|
||
|
|
Mean (± SD)
|
|
| 45 to 60 years old (n=12)
|
≥70 years old (n=12)
|
|
| AUC (ng•hr/mL)
|
389 (98)
|
463 (186)
|
| Peak Concentration (ng/mL)
|
46.2 (8.7)
|
48.4 (14.7)
|
| Time to Peak (hours)
|
1.8 (0.7)
|
1.8 (0.6)
|
| Half-Life (hours)*
|
6 (1.5)
|
8.2 (2.5)
|
Clinical Studies
FINASTERIDE INDICATIONS AND USAGE
FINASTERIDE CONTRAINDICATIONS
Pregnancy.
WARNINGS, EXPOSURE OF WOMEN — RISK TO MALE FETUS PRECAUTIONS, Information for Patients Pregnancy
WARNINGS
PRECAUTIONS, Pediatric Use WARNINGS, EXPOSURE OF WOMEN — RISK TO MALE FETUS; PRECAUTIONS , Information for Patients Pregnancy HOW SUPPLIED
PRECAUTIONS
General
Information for Patients
CONTRAINDICATIONS ; , WARNINGS, EXPOSURE OF WOMEN — RISK TO MALE FETUS; PRECAUTIONS Pregnancy HOW SUPPLIED
ADVERSE REACTIONS
ADVERSE REACTIONS
Drug/Laboratory Test Interactions
Drug Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
(0-24 hr)(0-24 hr)
in vitroin vitroin vitroin vivo
Pregnancy
. CONTRAINDICATIONS
in utero
11in utero
in utero
Nursing Mothers
Pediatric Use
Geriatric Use
CLINICAL PHARMACOLOGY, Pharmacokinetics Clinical Studies
FINASTERIDE ADVERSE REACTIONS
OVERDOSAGE
222
FINASTERIDE DOSAGE AND ADMINISTRATION
CLINICAL PHARMACOLOGY, Pharmacokinetics
Finasteride Tablets, USP
Patient Information about Finasteride Tablets
Finasteride tablets are for use by men only.
What are finasteride tablets?
Who should NOT take finasteride tablets?
Do Not Take finasteride tablets if you are:
- a woman who is pregnant or may potentially be pregnant. Finasteride tablets may harm your unborn baby. Do not touch or handle crushed or broken finasteride tablets (see ). “A warning about finasteride tablets and pregnancy”
- allergic to finasteride or any of the ingredients in finasteride tablets. See the end of this leaflet for a complete list of ingredients in finasteride tablets.
A warning aboutfinasteride tablets and pregnancy.
How should I take
finasteride tablets?
- Take one tablet by mouth each day. To avoid forgetting to take finasteride tablets, you can take them at the same time every day.
- If you forget to take finasteride tablets, do not take an extra tablet. Just take the next tablet as usual.
- You may take finasteride tablets with or without food.
- Do not share finasteride tablets with anyone else; they were prescribed only for you.
What are the possible side effects offinasteride tablets?
- trouble getting or keeping an erection (impotence)
- decrease in sex drive
- decreased volume of ejaculate
- ejaculation disorders
- enlarged or painful breast. You should promptly report to your doctor any changes in your breasts such as lumps, pain or nipple discharge.
Allergic reactions:
Testicular pain
:
What you need to know while taking finasteride tablets?
- Follow your doctor's advice about when to have these checkups. You should see your doctor regularly while taking finasteride tablets.
- Your doctor has prescribed finasteride tablets for BPH and not for treatment of prostate cancer — but a man can have BPH and prostate cancer at the same time. Checking for prostate cancer should continue while you take finasteride tablets. Checking for prostate cancer.
- Your doctor may have done a blood test called PSA for the screening of prostate cancer. Because finasteride tablets decreases PSA levels, you should tell your doctor(s) that you are taking finasteride tablets. Changes in PSA levels will need to be carefully evaluated by your doctor(s). Any increase in follow-up PSA levels from their lowest point should be carefully evaluated, even if the test results are still within the normal range. You should also tell your doctor if you have not been taking finasteride tablets as prescribed because this may affect the PSA test results. For more information, talk to your doctor. About Prostate-Specific Antigen (PSA).
How should I store finasteride tablets?
- Store finasteride tablets in a dry place at room temperature.
- Keep finasteride tablets in the original container and keep the container closed.
Finasteride tablets are coated and will prevent contact with the active ingredient during normal handling, provided that the tablets are not broken or crushed.
What are the ingredients in
finasteride tablets?Active ingredient:
Inactive ingredients:
What is BPH?
- a weak or interrupted urinary stream
- a feeling that you cannot empty your bladder completely
- a feeling of delay or hesitation when you start to urinate
- a need to urinate often, especially at night
- a feeling that you must urinate right away.
What
finasteride tablets do?
- Even though the prostate shrinks, you may NOT notice an improvement in urine flow or symptoms.
- You may need to take finasteride tablets for six (6) months or more to see whether it improves your symptoms.
- Therapy with finasteride tablets may reduce your risk for a sudden inability to pass urine and the need for surgery for an enlarged prostate.
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
Finasteride 5mg Tablet

FinasterideFinasteride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!