Fluconazole
FULL PRESCRIBING INFORMATION: CONTENTS*
- FLUCONAZOLE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS AND METABOLISM
- MICROBIOLOGY
- INDICATIONS & USAGE
- CLINICAL STUDIES
- FLUCONAZOLE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- FLUCONAZOLE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
FLUCONAZOLE DESCRIPTION
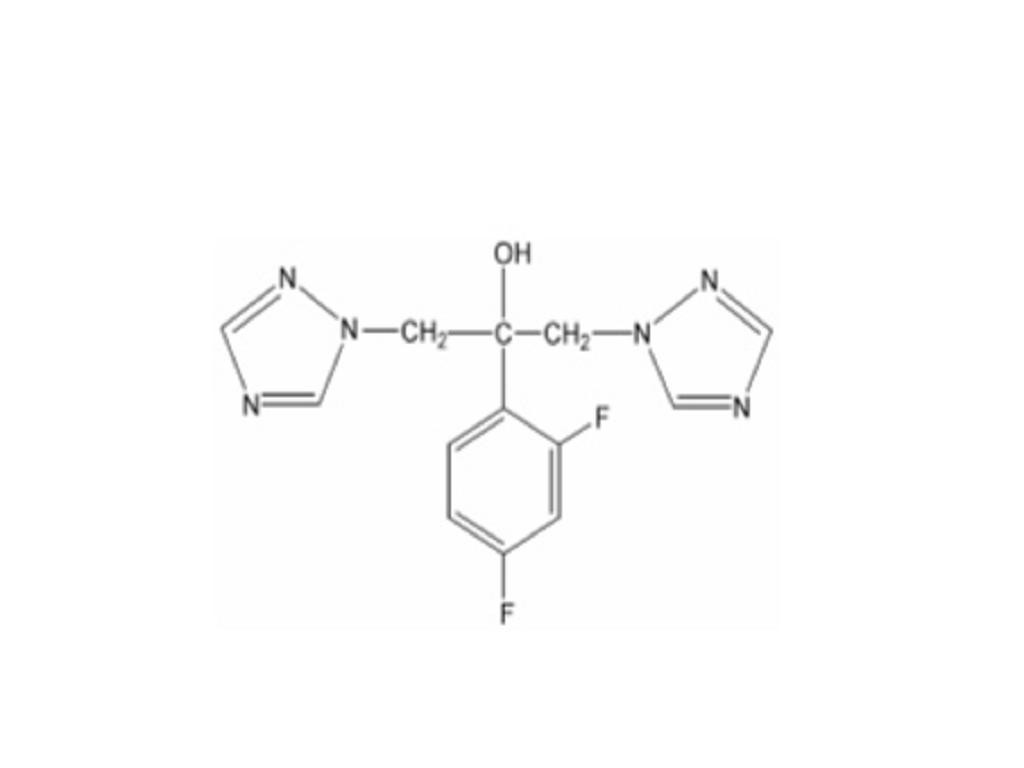
CLINICAL PHARMACOLOGY
PHARMACOKINETICS AND METABOLISM
Pharmacokinetics and MetabolismDOSAGE AND ADMINISTRATION
**
DOSAGE AND ADMINISTRATION
Pharmacokinetics in Children
Pharmacokinetics in Elderly
Drug Interaction Studies
Oral Contraceptives
Cimetidine
Antacid
1
1
Hydrochlorothiazide
Rifampin
PRECAUTIONS
Warfarin
PRECAUTIONS
Phenytoin
PRECAUTIONS
Cyclosporine
PRECAUTIONS
Zidovudine
Theophylline
PRECAUTIONS
Terfenadine
CONTRAINDICATIONSPRECAUTIONS
Oral Hypoglycemics
PRECAUTIONS
Tolbutamide
PRECAUTIONS
Glipizide
PRECAUTIONS
Glyburide
PRECAUTIONS
Rifabutin
PRECAUTIONS
Tacrolimus
PRECAUTIONS
Cisapride
CONTRAINDICATIONSPRECAUTIONS
Midazolam
PRECAUTIONS
Azithromycin
MICROBIOLOGY
Mechanism of ActionActivity In Vitro and In Clinical Infections
2
2
Table 1Dosage and Administration
Susceptibility Testing Methods
Cryptococcus Neoformans and Filamentous Fungi
Candida Species: Broth Dilution Techniques
Diffusion Techniques
**
Quality Control
**
Activity In Vivo
Drug Resistance
INDICATIONS & USAGE
CLINICAL STUDIES
CLINICAL STUDIES
Cryptococcal MeningitisVaginal Candidiasis
Pediatric Studies
Oropharyngeal Candidiasis
**
FLUCONAZOLE CONTRAINDICATIONS
CLINICAL PHARMACOLOGY: Drug Interaction StudiesPRECAUTIONSWARNINGS
PRECAUTIONS
GeneralSingle Dose
ADVERSE REACTIONSCLINICAL STUDIES
DRUG INTERACTIONS
CLINICAL PHARMACOLOGY: Drug Interaction StudiesCONTRAINDICATIONSOral Hypoglycemics
CLINICAL PHARMACOLOGY: Drug Interaction Studies
Coumarin-Type Anticoagulants
CLINICAL PHARMACOLOGY: Drug Interaction Studies
Phenytoin
CLINICAL PHARMACOLOGY: Drug Interaction Studies
Cyclosporine
CLINICAL PHARMACOLOGY: Drug Interaction Studies
Rifampin
CLINICAL PHARMACOLOGY: Drug Interaction Studies
Theophylline
CLINICAL PHARMACOLOGY: Drug Interaction Studies
Terfenadine
CONTRAINDICATIONSCLINICAL PHARMACOLOGY: Drug Interaction Studies
Cisapride
CONTRAINDICATIONSCLINICAL PHARMACOLOGY: Drug Interaction Studies
Astemizole
Rifabutin
CLINICAL PHARMACOLOGY: Drug Interaction Studies
Tacrolimus
CLINICAL PHARMACOLOGY: Drug Interaction Studies
Short-Acting Benzodiazepines
CLINICAL PHARMACOLOGY: Drug Interaction Studies
CLINICAL PHARMACOLOGY: Drug Interaction Studies
CLINICAL PHARMACOLOGY
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CLINICAL PHARMACOLOGY
PREGNANCY
Teratogenic Effects. Pregnancy Category CNURSING MOTHERS
PEDIATRIC USE
CLINICAL STUDIESCLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
ADVERSE REACTIONS
CLINICAL PHARMACOLOGY
GERIATRIC USE
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
FLUCONAZOLE ADVERSE REACTIONS
In Patients Receiving a Single Dose for Vaginal CandidiasisIn Patients Receiving Multiple Doses for Other Infections
Hepatobiliary
WARNINGS
Post-Marketing Experience
Immunologic
Cardiovascular
PRECAUTIONS
Central Nervous System
Dermatologic
WARNINGS
Hematopoietic and Lymphatic
Metabolic
Gastrointestinal
Other Senses
Adverse Reactions in Children
OVERDOSAGE
DOSAGE & ADMINISTRATION
Dosage and Administration in AdultsSingle Dose
Vaginal Candidiasis
Multiple Dose
Oropharyngeal Candidiasis
Esophageal Candidiasis
Systemic Candida Infections
Urinary Tract Infections and Peritonitis
Cryptococcal Meningitis
Prophylaxis in Patients Undergoing Bone Marrow Transplantation
Dosage and Administration in Children
**CLINICAL PHARMACOLOGY
Oropharyngeal Candidiasis
Esophageal Candidiasis
Systemic Candida Infections
Cryptococcal Meningitis
Dosage In Patients With Impaired Renal Function
HOW SUPPLIED
STORAGE AND HANDLING
REFERENCES
INFORMATION FOR PATIENTS
FLUCONAZOLE TABLETS, USP-
● itching
-
● a burning feeling when you urinate
-
● redness
-
● soreness
-
● a thick white vaginal discharge that looks like cottage cheese
-
● diabetes medicines you take by mouth such as glyburide, tolbutamide, glipizide
-
● blood thinners such as warfarin
-
● cyclosporine (used to prevent rejection of organ transplants)
-
● rifampin or rifabutin (used for tuberculosis)
-
● astemizole (used for allergies)
-
● tacrolimus (used to prevent rejection of organ transplants)
-
● phenytoin (used for seizures)
-
● theophylline (used for asthma)
-
● cisapride (Propulsid
-
● terfenadine (Seldane
-
● are taking any over-the-counter medicines you can buy without a prescription, including natural or herbal remedies
-
● have any liver problems
-
● have any other medical conditions
-
● are pregnant, plan to become pregnant, or think you might be pregnant. Your doctor will discuss whether fluconazole is right for you.
-
● are breast-feeding. Fluconazole can pass through breast milk to the baby.
-
● are allergic to any other medicines including those used to treat yeast and other fungal infections
-
● are allergic to any of the ingredients in fluconazole. The main ingredient of fluconazole tablets is fluconazole. If you need to know the inactive ingredients, ask your doctor or pharmacist.
-
● headache
-
● diarrhea
-
● nausea or upset stomach
-
● dizziness
-
● stomach pain
-
● changes in the way food tastes
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

FluconazoleFluconazole TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!