Fluconazole
FULL PRESCRIBING INFORMATION: CONTENTS*
- FLUCONAZOLE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- CLINICAL STUDIES
- FLUCONAZOLE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- FLUCONAZOLE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- REFERENCES
- SPL PATIENT PACKAGE INSERT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
FLUCONAZOLE DESCRIPTION
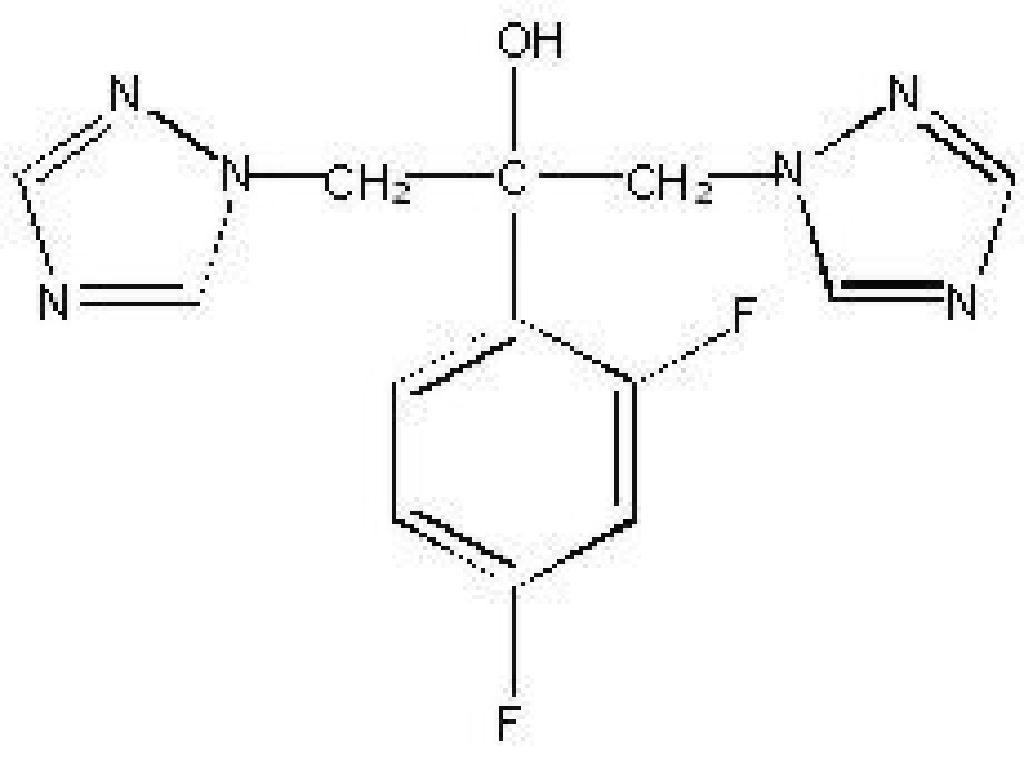
CLINICAL PHARMACOLOGY
Pharmacokinetics and MetabolismTissue or FluidRation of Fluconazole (Fluid)/Plasma Concentration*DOSAGE AND ADMINISTRATION
Pharmacokinetics in Children
AgeDoseClearanceHalf-lifeCmaxVdssStudied(mg/kg)(mL/min/kg)(Hours)(ug/mL)(L/kg)
Pharmacokinetics in Elderly
Drug Interaction Studies
PRECAUTIONS
PRECAUTIONS
PRECAUTIONS
PRECAUTIONS
PRECAUTIONS
CONTRAINDICATIONSPRECAUTIONS
PRECAUTIONS
PRECAUTIONS
PRECAUTIONS
PRECAUTIONS.
PRECAUTIONS
PRECAUTIONS
CONTRAINDICATIONSPRECAUTIONSPRECAUTIONS
Microbiology
Table 1Dosage and Administration
Susceptibility Testing Methods
+
+
Quality Control
Activity In Vivo
Drug Resistance
INDICATIONS & USAGE
CLINICAL STUDIES
CLINICAL STUDIES
Fluconazole POVaginal Product qhs150mg tabletx 7 days
ParameterFluconazole POVaginal Products
FluconazoleNystatinEnrolled9690Clinical Cure76/88 (86%)36/78 (46%)Mycological55/72 (76%)6/54 (11%)eradication**Subjects without follow-up cultures for any reason were considered nonevaluable for mycological response.The proportion of patients with clinical relapse 2 weeks after the end of treatment was 14% for subjects receiving fluconazole and 16% for subjects receiving nystatin. At 4 weeks after the end of treatment the percentages of patients with clinical relapse were 22% for fluconazole and 23% for nystatin.
FLUCONAZOLE CONTRAINDICATIONS
CLINICAL PHARMACOLOGY: Drug Interaction StudiesPRECAUTIONSWARNINGS
1. Hepatic injury: Fluconazole has been associated with rare cases of serious hepatic toxicity, including fatalities primarily in patients with serious underlying medical conditions. In cases of fluconazole-associated hepatotoxicity, no obvious relationship to total daily dose, duration of therapy, sex or age of the patient has been observed. Fluconazole hepatotoxicity has usually, but not always, been reversible on discontinuation of therapy. Patients who develop abnormal liver function tests during fluconazole therapy should be monitored for the development of more severe hepatic injury. Fluconazole should be discontinued if clinical signs and symptoms consistent with liver disease develop that may be attributable to fluconazole.PRECAUTIONS
GeneralADVERSE REACTIONSCLINICAL STUDIES
Interactions
Drug Interactions
CLINICAL PHARMACOLOGY: Drug Interaction StudiesCONTRAINDICATIONS
CLINICAL PHARMACOLOGY: Drug Interaction Studies
CLINICAL PHARMACOLOGY: Drug Interaction Studies
CLINICAL PHARMACOLOGY: Drug Interaction Studies
CLINICAL PHARMACOLOGY: Drug Interaction Studies
CLINICAL PHARMACOLOGY: Drug Interaction Studies
CLINICAL PHARMACOLOGY: Drug Interaction Studies
CONTRAINDICATIONSCLINICAL PHARMACOLOGY: Drug Interaction Studies
CONTRAINDICATIONSCLINICAL PHARMACOLOGY: Drug Interaction Studies
CLINICAL PHARMACOLOGY: Drug Interaction Studies
CLINICAL PHARMACOLOGY: Drug Interaction Studies
CLINICAL PHARMACOLOGY: Drug Interaction StudiesCLINICAL PHARMACOLOGY: Drug Interaction Studies
CLINICAL PHARMACOLOGY
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CLINICAL PHARMACOLOGY
PREGNANCY
Teratogenic EffectsPregnancy Category C:
There are no adequate and well controlled studies in pregnant women. There have been reports of multiple congenital abnormalities in infants whose mothers were being treated for 3 or more months with high dose (400-800 mg/day) fluconazole therapy for coccidioidomycosis (an unindicated use). The relationship between fluconazole use and these events is unclear. Fluconazole should be used in pregnancy only if the potential benefit justifies the possible risk to the fetus.
NURSING MOTHERS
PEDIATRIC USE
CLINICAL STUDIESCLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
ADVERSE REACTIONS
CLINICAL PHARMACOLOGY
GERIATRIC USE
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
FLUCONAZOLE ADVERSE REACTIONS
WARNINGS
PRECAUTIONS
WARNINGS
FluconazoleComparative Agents(N = 577)(N = 451)
OVERDOSAGE
DOSAGE & ADMINISTRATION
Pediatric PatientsAdultsCLINICAL PHARMACOLOGY
Creatinine Clearance (mL/min)Percent of Recommended Dose
Weight (kg) x (140-age)
STORAGE AND HANDLING
REFERENCES
SPL PATIENT PACKAGE INSERT
PATIENT INFORMATIONPACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION



FluconazoleFluconazole TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!