Fludeoxyglucose
Cyclotron Partners LP dba Cyclotope
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Fludeoxyglucose F18 Injection safely and effectively. See full prescribing information for Fludeoxyglucose F18 Injection. Fludeoxyglucose F18 Injection Initial U.S. Approval: 2005DOSAGE FORMS AND STRENGTHSMultiple-dose glass vial containing 0.74-18.5 GBq/mL (20-500 mCi/mL) of Fludeoxyglucose F18 Injection and 4.5 mg of sodium chloride in citrate buffer (approximately 16 – 17 mL volume), for intravenous administration(3). Side EffectsHypersensitivity reactions have occurred; have emergency resuscitation equipment and personnel immediately available (6). To report SUSPECTED ADVERSE REACTIONS, contact CYCLOTOPE at 1-713-747-5686 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
FULL PRESCRIBING INFORMATION: CONTENTS*
- 3 DOSAGE FORMS AND STRENGTHS
- 6 FLUDEOXYGLUCOSE ADVERSE REACTIONS
- 11 FLUDEOXYGLUCOSE DESCRIPTION
- 16 HOW SUPPLIED / STORAGE AND DRUG HANDLING
FULL PRESCRIBING INFORMATION
3 DOSAGE FORMS AND STRENGTHS
Multiple-dose glass vial containing 0.74-18.5 GBq/mL (20-500 mCi/mL) of Fludeoxyglucose F 18 Injection and 4.5 mg of sodium chloride in citrate buffer (approximately 16 - 17 mL volume) for intravenous administration.
6 ADVERSE REACTIONS
Hypersensitivity reactions with pruritus, edema and rash have been reported in the post-marketing setting. Have emergency resuscitation equipment and personnel immediately available.
11 DESCRIPTION
11.1 Chemical Characteristics
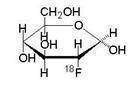
Fludeoxyglucose F 18 Injection is a positron emitting radiopharmaceutical that is used for diagnostic purposes in conjunction with positron emission tomography (PET) imaging. The active ingredient 2-deoxy-2-[18F]fluoro-D-glucose has the molecular formula of C6H11 18FO5with a molecular weight of 181.26, and has the following chemical structure:
Fludeoxyglucose F 18 Injection is provided as a ready to use sterile, pyrogen free, clear, colorless citrate buffered solution. Each mL contains between 0.740 to 18.5 GBq (20.0-500 mCi) of 2-deoxy-2-[18F]fluoro-D-glucose at the EOS, 4.5 mg of sodium chloride in citrate buffer. The pH of the solution is between 4.5 and 7.5. The solution is packaged in a multiple-dose glass vial and does not contain any preservative.
11.2 Physical Characteristics
Fluorine F 18 has a physical half-life of 109.7 minutes and decays to Oxygen O 18 (stable) by positron decay. The principal photons useful for imaging are the dual 511 keV "annihilation" gamma photons, that are produced and emitted simultaneously in opposite direction when the positron interacts with an electron (Table 2).
| Radiation/Emission | % per Disintegration | Mean Energy |
|---|---|---|
| Positron (β+) | 96.73 | 249.8 keV |
| Gamma (±) From: Kocher, D.C. Radioactive Decay Tables DOE/TIC-I 1026, 89 (1981) |
193.46 | 511.0 keV |
The specific gamma ray constant (point source air kerma coefficient) for fluorine F 18 is 5.7 R/hr/mCi (1.35 x 10 -6 Gy/hr/kBq) at 1 cm. The half-value layer (HVL) for the 511 keV photons is 4 mm lead (Pb). The range of attenuation coefficients for this radionuclide as a function of lead shield thickness is shown in Table 3. For example, the interposition of an 8 mm thickness of Pb, with a coefficient of attenuation of 0.25, will decrease the external radiation by 75%.
| Shield Thickness (Pb) mm | Coefficient of Attenuation |
|---|---|
| 0 | 0.00 |
| 4 | 0.50 |
| 8 | 0.25 |
| 13 | 0.10 |
| 26 | 0.01 |
| 39 | 0.001 |
| 52 | 0.0001 |
For use in correcting for physical decay of this radionuclide, the fractions remaining at selected intervals after calibration are shown in Table 4.
| Minutes | Fraction Remaining |
|---|---|
| 0 |
1.00 |
| 15 | 0.909 |
| 30 | 0.826 |
| 60 | 0.683 |
| 110 | 0.500 |
| 220 | 0.250 |
16 HOW SUPPLIED / STORAGE AND DRUG HANDLING
Fludeoxyglucose F 18 Injection is supplied in a multi-dose, capped 30 mL glass vial containing between 0.740 – 18.5 GBq/mL (20 - 500 mCi/mL), of no carrier added 2-deoxy-2-[F 18] fluoro-D-glucose, at end of synthesis, in approximately 16 - 17 mL. The contents of each vial are sterile, pyrogen-free and preservative-free.
NDC 47584-001-01
Store the Fludeoxyglucose F 18 Injection vial upright in a lead shielded container at 20º to 25°C (68º to 77°F); excursions permitted to 15-30°C (59-86°F) [See USP Controlled Room Temperature].
Distribute, store and dispose of Fludeoxyglucose F 18 Injection in accordance with the regulations and a general license, or its equivalent, of an Agreement State or a Licensing State.
The expiration date and time are provided on the container label. Use Fludeoxyglucose F 18 Injection within 12 hours from the EOS time.
Label for Container Closure System: 30 ml Vial

Label for Lead Pig Container

|
Manufactured and distributed by: Cyclotope Accelerated Medicine 8285 El Rio Suite 160 Houston, TX 77054 USA |
FludeoxyglucoseFludeoxyglucose F18 INJECTION
| |||||||||||||||||||||||||||||||||||||||||||||||||