Flumazenil
General Injectables & Vaccines, Inc
Flumazenil Injection
FULL PRESCRIBING INFORMATION: CONTENTS*
- FLUMAZENIL DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL TRIALS
- INDIVIDUALIZATION OF DOSAGE
- INDICATIONS & USAGE
- FLUMAZENIL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- FLUMAZENIL ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL
FULL PRESCRIBING INFORMATION
FLUMAZENIL DESCRIPTION
Flumazenil injection is a benzodiazepine receptor antagonist. Chemically, flumazenil is ethyl 8-fluoro-5, 6-oxo-4H-imidazo [1,5-a](1,4) benzodiazepine-3-carboxylate. Flumazenil has an imidazobensodiazepine structure, a calculated molecular weight of 303.3 and the following structural formula:
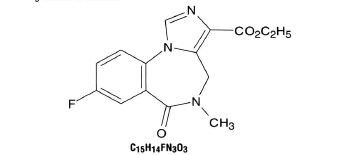
Flumazenil is a white to off-white crystalline compound with an octanol:buffer partition coefficient of 14 to 1 at pH 7.4. It is insoluble in water but slightly soluble in acidic aqueous solutions. Flumazenil injection is available as a sterile parenteral dosage form for intravenous administration. Each mL contains flumazenil 0.1 mg. In addition, the following inactive ingredients are included: methylparaben 1.8 mg. propyparaben 0.2mg, sodium chloride 0.9%, edetate disodium 0.01%, acetic acid 0.01%, hydrochloric acid and/or sodium hydroxide to adjust pH to 3.5 to 4.5, and water for injection.
CLINICAL PHARMACOLOGY
Flumazenil, an imidazobenzodiazepine derivative, antagonizws the actions of benzodiazepines on the central nervous system. Flumazenil competitvely inhibits the activity at the benzodiazepine recognition site on the GABA/benzodiazepine receptor complex. Glumazenil is a weak partial agonist in some animal model of activity, but has little or no agonist activity in man.
Flumazenil does not antagonize the central nervous system effects of drug affecting GABA-ergic neurons by means other than the benzodiazepine receptor (including ethanol, barbiturates, or general anesthetics) and does not reverse the effects of opioids.
In animals pretreated with high doses of benzodiazepines over several weeks, flumazenil elicited symptoms of benzodiazepine withdrawal, including seizures. A similar effect was seen in adult human subjects.
Intravenous flumazenil has been shown to antagonize
sedation, impairment of recall, psychomotor impairment and ventilatory
depression produced by benzodiazepines in healthy human volunteers.
The duration and degree of reversal of sedative benzodiazepine effects are related to the dose and plasma concentrations of flumazenil as shown in the following data from a study in normal volunteers.
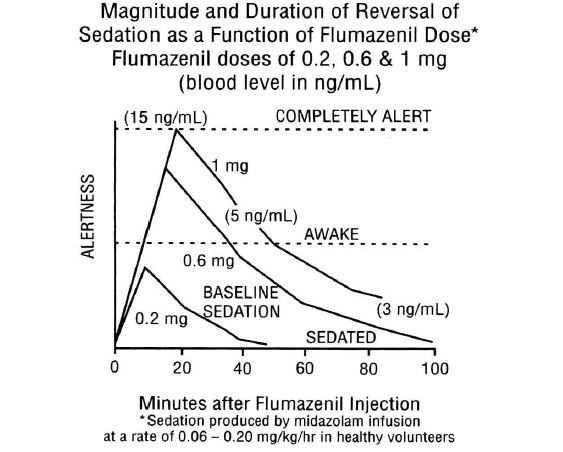
Generally, doses of approximately 0.1 mg to 0.2 mg (corresponding to peak plasma levels of 3 to 6 ng/mL) produce partial antagonism, whereas higher doses of 0.4 to 1mg (peak plasma levels of 12 to 28 ng/mL) usually produce complete antagonism in patients who have received the usual sedating doses of benzodiazepines. The onset of reversal is usually evident within 1 to 2 minutes after the injection is completed. Eighty percent response will be reached within 3 minutes, with the peak effect occurring at 6 to 10 minutes. The duration and degree of reversal are related to the plasma concentration of the sedating benzodiazepine as well as the dose of flumazenil given.
In healthy volunteers, flumazenil did not alter intraocular pressure when given alone and reversed the decrease in intraocular pressure seen agter administration of midazolam.
Pharmacokinetics:
After IV administration, plasma concentrations of flumazenil follow a two-exponential decay model. The pharmacokinetics of flumazenil are dose-proportional up to 100 mg.
Distribution:
Flumazenil is extensively distributed in the extravascular space with an initial distribution half-life of 4 to 11 minutes and a terminal half-life of 40 to 80 minutes. Peak concentrations of flumazenil are proportional to dose, with an apparent initial volume of distribution of 0.5 L/kg. The volume of distribution at steady-state is 0.9 to 1.1L/kg. Flumazenil is a weak lipophilic base. Protein binding is approximately 50% and the drug shows no preferential partitioning into red blood cells. Albumin accounts for two thirds of plasma protein binding.
Metabolism:
Flumazenil is completely (99%) metabolized. Very little unchanged flumazenil (less than 1%) is found in the urine. The major metabolites of flumazenil identified in urine are the de-ethylated free acid and its glucuronide conjugate. In preclinical studies there was no evidence of pharmacologic activity exhibited by the de-ethylated free acid.
Elimination:
Elimination of radiolabeled drug is essentially complete within 72 hours, with 90% to 95% of the radioactivity appearing in urine and 5% to 10% in the feces. Clearance of flumazenil occurs primarily by hepatic metabolism and is dependent on hepatic blood flow. In pharmacokinetic studies of normal volunteers, total clearance ranged from 0.8 to 1.0 L/hr/kg.
Pharmacokinetic parameters following a 5-minute infusion of a total of 1mg of flumazenil mean (coefficient of variation, range):
| Cmax (ng/mL) |
24 |
(38%, 11-43) |
| AUC (ng-hr/mL0 |
15 |
(22%, 10-22) |
| Vss (L/kg) |
1 |
(24%, 0.8-1.6) |
| CI (L/hr/kg) |
1 |
(20%, 0.7-1.4) |
| Half-life (min) |
54 |
(21%, 41-79) |
Food Effects:
Ingestion of food during an intravenous infusion of the drug results in a 50% increase in clearance, most likely due to the increased hepatic blood flow that accompanies a meal.
Special Populations:
The Elderly:
The pharmacokinetics of flumazenil are not significantly altered in the elderly.
Gender:
The pharmacokinetics of flumazenil are not different in male and female subjects.
Renal Failure (creatinine clearance less than 10mL/min) and Hemodialysis:
The pharmacokinetics of flumazenil are not significantly affected.
Patients With Liver Dysfunction:
For patients with moderate liver dysfunction, their mean total clearance is decreased to 40% to 60% and in patients with severe liver dysfunction, it is decreased to 25% of normal value, compared with age-matched healthy subject. This results in a prolongation of the half-life to 1.3 hours in patients with moderate hepatic impairment and 2.4 hours in severely impaired patients. Caution should be exercised with initial and /or repeated dosing to patients with liver disease.
Drug-Drug Interaction:
The pharmacokinetic profile of flumazenil is unaltered in the presence of benzodiazepine agonists and the kinetic profiles of those benzodiazepines studied (i.e., diazepam, flunitrazepam, lormetazepam, and midazolam) are unaltered by flumazenil. During the 4-hour steady-state and post infusion of ethanol, there were no pharmacokinetic interactions on ethanol mean plasma levels as compared to placebo when flumazenil doses were given intravenously (at 2.5 hours and 6 hours) nor were interactions of ethanol on the flumazenil elimination half-life found.
Pharmacokinetics in Pediatric Patients:
The pharmacokinetics of flumazenil have been evaluated in 29 pediatric patients ranging in age from 1 to 17 years who had undergone minor surgical procedures. The average doses administered were 0.53 mg (0.044 mg/kg) in patients aged 1 to 5 years, 0.63 mg (0.020 mg/kg) in patients aged 6 to 12 years, and 0.8 mg (0.014 mg/kg) in patients aged 13 to 17 years. Compared to adults, the elimination half-life in pediatric patients was more variable, averaging 40 minutes (range: 20 to 75 minutes). Clearance and volume of distribution, normalized for body weight, were in the same range as those seen in adults, although more variability was seen in the pediatric patients.
CLINICAL TRIALS
Conscious Sedation in Adults:
PRECAUTIONS
General Anesthesia in Adults:
PRECAUTIONS
Management of Suspected Benzodiazepine Overdose in Adults:
WARNINGS
INDIVIDUALIZATION OF DOSAGE
General Principles:
The serious adverse effects of flumazenil are related to the reversal of benzodiazepine effects. Using more than the minimally effective dose of flumazenil is tolerated by most patients but may complicate the management of patients who are physically dependent on benzodiazepines or patients who are depending on benzodiazepines for therapeutic effect (such as suppression of seizures in cyclic antidepressant overdose).
In high-risk patients, it is important to administer the smallest amount of flumazenil injection that is effective. The 1-minute wait between individual doses in the dosetitration recommended for general clinical populations may be too short for high-risk patients. This is because it takes 6 to 10 minutes for any single dose of flumazenil to reach full effects. Practitioners should slow the rate of administration of flumazenil injection administered to high-risk patients as recommended below.
Anesthesia and Conscious Sedation in Adult Patients:
Flumazenil is well tolerated at the recommended doses in individuals who have no tolerance to (or dependence on) benzodiazepines. The recommended doses and titration rates in anesthesia and conscious sedation (0.2 mg to 1 mg given at 0.2 mg/min) are well tolerated in patients receiving the drug for reversal of a single benzodiazepine exposure in most clinical settings (see ADVERSE REACTIONS). The major risk will be resedation because the duration of effect of a long-acting (or large dose of a short-acting) benzodiazepine may exceed that of flumazenil injection. Resedation may be treated by giving a repeat dose at no less than 20-minute intervals. For repeat treatment, no more than 1 mg (at 0.2 mg/min doses) should be given at any one time and no more than 3 mg should be given in any one hour.
Benzodiazepine Overdose in Adult Patients:
The risk of confusion, agitation, emotional lability and perceptual distortion with the doses recommended in patients with benzodiazepine overdose (3 mg to 5 mg administered as 0.5 mg/min) may be greater than that expected with lower doses and slower administration. The recommended doses represent a compromise between a desirable slow awakening and the need for prompt response and a persistent effect in the overdose situation. If circumstances permit, the physician may elect to use the 0.2 mg/minute titration rate to slowly awaken the patient over 5 to 10 minutes, which may help to reduce signs and symptoms on emergence.
Flumazenil has no effect in cases where benzodiazepines are not responsible for sedation. Once doses of 3 mg to 5 mg have been reached without clinical response, additional flumazenil is likely to have no effect.
Patients Tolerant to Benzodiazepines:
Flumazenil injection may cause benzodiazepine withdrawal symptoms in individuals who have been taking benzodiazepines long enough to have some degree of tolerance. Patients who had been taking benzodiazepines prior to entry into the flumazenil trials, who were given flumazenil in doses over 1 mg, experienced withdrawal-like events 2 to 5 times more frequently than patients who received less than 1 mg.
In patients who may have tolerance to benzodiazepines, as indicated by clinical history or by the need for larger than usual doses of benzodiazepines, slower titration rates of 0.1 mg/min and lower total doses may help reduce the frequency of emergent confusion and agitation. In such cases, special care must be taken to monitor the patients for resedation because of the lower doses of flumazenil injection used.
Patients Physically Dependent on Benzodiazepines:
Flumazenil is known to precipitate withdrawal seizures in patients who are physically dependent on benzodiazepines, even if such dependence was established in relatively few days of high-dose sedation in Intensive Care Unit (ICU) environments. The risk of either seizures or resedation in such cases is high and patients have experienced seizures before regaining consciousness. Flumazenil injection should be used in such setting with extreme caution, since the use of flumazenil in this situation has not been studied and no information as to dose and rate of titration is available. Flumazenil injection should be used in such patients only if the potential benefits of using the drug outweigh the risks of precipitated seizures. Physicians are directed to the scientific literature for the most current information in this area.
INDICATIONS & USAGE
Adult Patients:
Flumazenil injection is indicated for the complete of partial reversal of the sedative effects of benzodiazepines in cases where general anesthesia has been induced and/or maintained with benzodiazepines, where sedation has been produced with benzodiazepines for diagnostic and therapeutic procedures, and for the management of benzodiazepine overdose.
Pediatric Patients (aged 1 to 17):
Flumazenil injection is indicated for the reversal of conscious sedation induced with benzodiazepines (see PRECAUTIONS: Pediatric Use).
FLUMAZENIL CONTRAINDICATIONS
Flumazenil injection is contraindicated:
in patients with a known hypersensitivity to flumazenil of benzodiazepines.
in patients who have been given a benzodiazepine for control of a potentially life-threatening condition (eg, control of intracranial pressure or status epilepticus).
in patients who are showing signs of serious cyclic antidepressant overdose (see WARNINGS).
WARNINGS
THE USE OF FLUMAZENIL HAS BEEN ASSOCIATED WITH THE OCCURRENCE OF SEIZURES.
THESE ARE MOST FREQUENT IN PATIENTS WHO HAVE BEEN ON BENZODIAZEPINES FOR LONG-TERM SEDATION OR IN OVERDOSE CASES WHERE PATIENTS ARE SHOWING SIGNS OF SERIOUS CYCLIC ANTIDEPRESSANT OVERDOSE.
PRACTITIONERS SHOULD INDIVIDUALIZE THE DOSAGE OF FLUMAZENIL INJECTION AND BE PREPARED TO MANAGE SEIZURES.
Risk of Seizures:
The reversal of benzodiazepine effects may be associated with the onset of seizures in certain high-risk populations. Possible risk factors for seizures include: concurrent major sedative-hypnotic drug withdrawal, recent therapy with repeated doses of parenteral benzodiazepines, myoclonic jerking or seizure activity prior to flumazenil administration in overdose cases, or concurrent cyclic antidepressant poisoning.
Flumazinil injection is not recommended in cases of serious cyclic antidepressant poisoning, as manifested by motor abnormalities (twitching, rigidity, focal seizure), dysrhythmia (wide QRS, ventricular dysrhythmia, heart block), anticholinergic signs (mydriasis, dry mucosa, hypoperistalsis), and cardiovascular collapse at presentation. In such cases flumazenil injection should be withheld and the patient should be allowed to remain sedated (with ventilatory and circulatory support as needed) until the signs of antidepressant toxicity have subsided. Treatment with flumazenil has no known benefit to the seriously ill mixed-overdose patient other than reversing sedation and should not be used in cases where seizures (from any cause) are likely.
Most convulsions associated with flumazenil administration require treatment and have been successfully managed with benzodiazepines, phenytoin or barbiturates. Because of the presence of flumazenil, higher than usual doses of benzodiazepines may be required.
Hypoventilation:
Patients who have received flumazenil injection for the reversal of benzodiazepine effects (after conscious sedation or general anesthesia) should be monitored for resedation, respiratory depression, or other residual benzodiazepine effects for an appropriate period (up to 120 minutes) based on the dose and duration of effect of the benzodiazepine employed.
This is because flumazenil has not been established in patients as an effective treatment for hypoventilation due to benzodiazepine administration. In healthy male volunteers, flumazenil is capable of reversing benzodiazepine-induced depression of the ventilatory responses to hypercapnia and hypoxia after a benzodiazepine alone. However, such depression may recur because the ventilatory effects of typical doses of flumazenil injection (1 mg or less) may wear off before the effects of many benzodiazepines. The effects of flumazenil on ventilatory response following sedation with a benzodiazepine in combination with an opioid are inconsistent and have not been adequately studied. The availability of flumazenil does not diminish the need for prompt detection of hypoventilation and the ability to effectively intervene by establishing and airway and assisting ventilation.
Overdose cases should always be monitored for resedation until the patients are stable and resedation is unlikely.
PRECAUTIONS
Return of Sedation:
Flumazenil injection may be expected to improve the alertness of patients recovering from a procedure involving sedation of anesthesia with benzodiazepines, but should not be substituted for an adequate period of postprocedure monitoring. The availability of flumazenil injection does not reduce the risks associated with the use of large doses of benzodiazepines for sedation.
Patients should be monitored for resedation, respiratory depression (see WARNING) or other persistent or recurrent agonist effects for an adequate period of time after administration of flumazenil injection.
Resedation is least likely in cases, where flumazenil is administered to reverse a low dose of a short-acting benzodiazepine (less than 10 mg midazolam). It is most likely in cases where a large single of cumulative dose of a benzodiazepine has been given in the course of a long procedure along with neuromuscular blocking agents and multiple anesthetic agents.
Profound resedation was observed in 1% to 3% of adult patients in the clinical studies. In clinical situations where resedation must be prevented in adult patients, physicians may wish to repeat the initial dose (up to 1 mg of flumazenil injection given at 0.2 mg/min) at 30 minutes and possibly again at 60 minutes. This dosage schedule, although not studied in clinical trials, was effective in preventing resedation in a pharmacologic study in normal volunteers.
The use of flumazenil to reverse the effects of benzodiazepines used for conscious sedation has been evaluated in one open-label clinical trial involving 107 pediatric patients between the ages of 1 and 17 years. This study suggested that pediatric patients who have become fully awake following treatment with flumazenil may experience a recurrence of sedation, especially younger patients (ages 1 to 5). Resedation was experienced in 7 of 60 patients who were fully alert 10 minutes after the start of flumazenil administration. No patient experienced a return to the baseline level of sedation. Mean time to resedation was 25 minutes (range: 19 to 50 minutes) (see PRECAUTIONS: Pediatric Use). The safety and effectiveness of repeated flumazenil administration in pediatric patients experiencing resedation have not been established.
Use in the ICU:
Flumazenil injection should be used with caution in the ICU because of the increased risk of unrecongnized benzodiazepine dependence in such settings. Flumazenil injection may produce convulsions in patients physically dependent on benzodiazepines (see INDIVIDUALIZATION OF DOSAGE AND WARNING).
Administration of flumazenil injection to diagnose benzodiazepine-induced sedation in the ICU is not recommended due to the risk of adverse events as described above. In addition, the prognostic significance of a patient's failure to respond to flumazenil in cases confounded by metabolic disorder, traumatic injury, drugs other than benzodiazepines, or any other reasons not associated with benzodiazepine receptor occupancy is unknown.
Use in Benzodiazepine Overdosage:
Flumazenil injection is intended as an adjunct to, not as a substitute for, proper management of airway, assisted breathing, circulatory access and support, internal decontamination by lavage and charcoal, and adequate clinical evaluation.
Necessary measures should be instituted to secure airway, ventilation and intravenous access prior to administering flumazenil. Upon arousal, patients may attempt to withdraw endotracheal tubes and/or intravenous lines as the result of confusion and agitation following awakening.
Head Injury:
Flumazenil injection should be used with caution in patients with head injury as it may be capable of precipitating convulsions or altering cerebral blood flow in patients receiving benzodiazepines. It should be used only by practitioners prepared to manage such complications should they occur.
Use with Neuromuscular Blocking Agents:
Flumazenil injection should not be used until the effects of neuromuscular blockade have been fully reversed.
Use in Psychiatric Patients:
Flumazenil has been reported to provoke panic attacks in patients with history of panic disorder.
Pain on Injection:
To minimize the likelihood of pain or inflammation at the injection site, flumazenil injection should be administered through a freely flowing intravenous infusion into a large vein. Local irritation may occur following extravasation into perivascular tissues.
Use in Respiratory Disease:
The primary treatment of patients with serious lung disease who experience serious respiratory depression due to benzodiazepines should be appropriate ventilatory support (see PRECAUTIONS) rather than the administration of flumazenil injection. Flumazenil is capable of partially reversing benzodiazepine-induced alterations in ventilatory drive in healthy volunteers, but has not been shown to be clinically effective.
Use in Cardiovascular Disease:
Flumazenil did not increase the work of the heart when used to reverse benzodiazepines in cardiac patients when given at a rate of 0.1 mg/min in total doses of less than 0.5 mg in studies reported in the clinical literature. Flumazenil alone had no significant effects on cardiovascular parameters when administered to patients with stable ischemic heart disease.
Use in Liver Disease:
The clearance of flumazenil is reduced to 40% to 60% of normal in patients with mild to moderate hepatic disease and to 25% of normal in patients with severe hepatic dysfunction (see CLINICAL PHARMACOLOGY: Pharmacokinetics). While the dose of flumazenil used for initial reversal of benzodiazepine effects is not affected, repeat doses of the drug in liver disease should be reduced in size of frequency.
Use in Drug-and Alcohol-Dependent Patients:
Flumazenil injection should be used with caution in patients with alcoholism and other drug dependencies due to the increased frequency of benzodiazepine tolerance and dependence observed in these patient populations.
Flumazenil injection is not recommended either as a treatment for benzodiazepine dependence or for the management of protracted benzodiazepine abstinence syndromes, as such use has not been studed.
The administration of flumazenil can precipitate benzodiazepine withdrawal in animals and man. This has been seen in healthy volunteers treated with therapeutic doses of oral lorazepam for up to 2 weeks who exhibited effects such as hot flushes, agitation and tremor when treated with cumulative doses of up to 3 mg doses of flumazenil.
Similar adverse experiences suggestive of flumazenil precipitation of benzodiazepine withdrawal have occurred in some adult patients in clinical trials. Such patients had a short-lived syndrome characterized by dizziness, mild confusion, emotional liability, agitation (with signs and symptoms of anxiety), and mild sensory distortions. This response was dose-related, most common at doses above 1 mg, rarely required treatment other than reassurance and was usually short lived. When required, these patients (5 to 10 cases) were successfully treated with usual doses of a barbiturate, a benzodiazepine, or other sedative drug.
Practitioners should assume that flumazenil administration may trigger dose-dependent withdrawal syndromes in patients with established physical dependence on benzodiazepines and may complicate the management of withdrawal syndromes for alcohol, barbiturates and cross-tolerant sedatives.
Drug Interactions:
Interaction with central nervous system depressants other than benzodiazepines has not been specifically studied; however, no deleterious interactions were seen when flumazenil was administered after narcotics, inhalational anesthetics, muscle relaxants and muscle relaxant antagonists administered in conjunction with sedation of anesthesia.
Particular caution is necessary when using flumazenil injection in cases of mixed drug overdosage since the toxic effects (such as convulsions and cardiac dysrhythmias) of other drugs taken in overdose (especially cyclic antidepressants) may emerge with the reversal of the benzodiazepine effect by flumazenil (see WARNINGS).
The use of flumazenil is not recommended in epileptic patients who have been receiving benzodiazepine treatment for a prolonged period. Although flumazenil exerts a slight intrinsic anticonvulsant effect, its abrupt suppression of the protective effect of a benzodiazepine agonist can give rise to convulsions in epileptic patients.
Flumazenil blocks the central effects of benzodiazepines by competitive interaction at the receptor level. The effects of nonbenzodiazepine agonists at benzodiazepine receptors, such as zopiclone, triazolopyridazines and others, are also blocked by flumazenil.
The pharmacokinetics of benzodiazepines are unaltered in the presence of flumazenil and vice versa.
There is no pharmacokinetic interaction between ethanol and flumazenil.
Use in Ambulatory Patients:
The effects of flumazenil injection may wear off before a long-acting benzodiazepoine is completely cleared from the body. In general, if a patient shows no signs of sedation within 2 hours after a 1 mg dose of flumazenil, serious resedation at a later time is unlikely. An adequate period of observation must be provided for any patient in whom either long-acting benzodiazepines (such as diazepam) or large doses of shortacting benzodiazepines (such as greater than 10 mg of midazolam) have been used (see INDIVIDUALIZATION OF DOSAGE).
Because of the increased risk of adverse reactions in patients who have been taking benzodiazepines on a regular basis, it is particularly important that physicians query patients or their guardians carefully about benzodiazepine, alcohol and sedative use as part of the history prior to any procedure in which the use of flumazenil injection is planned (see PRECAUTIONS:Use in Drug-and Alcohol-Dependent Patients).
Information for Patients:
Flumazenildoes not consistently reverse amnesia. Patients cannot e expected to remember information told to them in the postprocedure period and instructions given to patients should be reinforced in writing or given to a responsible family member. Physicians are advised to discuss with patients or their guardians, both before surgery and at discharge, that although the patient may feel alert at the time of discharge, the effects of the benzodiazepine (e.g., sedation) may recur. As a result, the patient should be instructed, preferably in writing, that their memory and judgment may be impaired and specifically advised:
1. Not to engage in any activities requiring complete alertness, and not to operate hazardous machinery or a motor vehicle during the first 24 hours after discharge, and it is certain no residual sedative effects of the benzodiazepine remain.
2. Not to take any alcohol or non-prescription drugs during the first 24 hours after flumazenil administration or if the effects of the benzodiazepine persist.
Laboratory Tests:
No specific laboratory tests are recommended to follow the patient's response or to identify possible adverse reactions.
Drug/Laboratory Test Interactions:
The possible interaction of flumazenil with commonly used laboratory tests has not been evaluated.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Carcinogenesis:
No studies in animals to evaluate the carcinogenic potential of flumazenil have been conducted.
Mutagenesis:
No evidence for mutagenicity was noted in the Ames test using five different tester strains. Assays for mutagenic potential in S. cerevisiae D7 and in Chinese hamster cells were considered to be negative as were blastogenesis assays in vitro in peripheral human lymphocytes and in vivo in a mouse micronucleus assay. Flumazenil caused a slight increase in unscheduled DNA synthesis in rat hepatocyte culture at concentrations which were also cytotoxic; no increase in DNA repair was observed in male mouse germ cells in an in vivo DNA repair assay.
Impairment of Fertility:
A reproduction study in male and female rats did not show any impairment of fertility at oral dosages of 125 mg/kg/day. From the available data on the area under the curve (AUC) in animals and man the dose represented 120 times the human exposure from a maximum recommended intravenous dose of 5 mg.
Pregnancy Category C:
There are no adequate and well-controlled studies of the use of flumazenil in pregnant women. Flumazenil should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Teratogenic Effects:
Flumazenil has been studied for teratogenicity in rats and rabbits following oral treatments of up to 150 mg/kg/day. The treatments during the major organogenesis were on days 6 to 15 of gestation in the rat and days 6to 18 of gestation in the rabbit. No teratogenic effects were observed in rats or rabbits at 150 mg/kg; the dose, based on the available data on the area under the plasma concentration-time curve (AUC) represented 120 times to 600 times the human exposure from a maximum recommended intravenous dose of 5 mg in humans. In rabbits, embryocidal effects (as evidenced by increased preimplantation and postimplantation losses) were observed at 50 mg/kg or 200 times the human exposure from a maximum recommended intravenous dose of 5 mg. The no-effect dose of 15 mg/kg in rabbits represents 60 times the human exposure.
Nonteratogenic Effects:
An animal reproduction study was conducted in rats at oral dosages of 5, 25, and 125 mg/kg/day of flumazenil. Pup survival was decreased during the lactating period, pup liver weight at weaning was increased for the high-dose group (125 mg/kg/day) and incisor eruption and ear opening in the offspring were delayed; the delay in ear opening was associated with a delay in the appearance of the auditory startle response. No treatment-related adverse effects were noted for the other dose groups. Based on the available data from AUC, the effect level (125 mg/kg) represents 120 times the human exposure from 5 mg, the maximum recommended intravenous dose in humans. The no-effect level represents 24 times the human exposure from an intravenous dose of 5 mg.
Labor and Delivery:
The use of flumazenil injection to reverse the effects of benzodiazepines used during labor and delivery is not recommended because the effects of the drug in the newborn are unknown.
Nursing Mothers:
Caution should be exercised when deciding to administer flumazenil injection to a nursing woman because it is not known whether flumazenil is excreted in human milk.
Pediatric Use:
The safety and effectiveness of flumazenil have been established in pediatric patients 1 year of age and older. Use of flumazenil injection in this age group is supported by evidence from adequate and well-controlled studies of flumazenil in adults with additional data from uncontrolled pediatric studies including one open-label trial.
The use of flumazenil to reverse the effects of benzodiazepines used for conscious sedation was evaluated in one uncontrolled clinical trial involving 107 pediatric patients between the ages of 1 and 17 years. At the doses used, flumazenil's safety was established in this population. Patients received up to 5 injections of 0.01 mg/kg flumazenil up to a maximum total dose of mg at a rate not exceeding 0.2 mg/min.
Of 60 patients who were fully alert at 10 minutes, 7 experienced resedation. Resedation occurred between 19 and 50 minutes after the start of flumazenil administration. None of the patients experienced a return to the baseline level of sedation. All 7 patients were between the ages of 1 and 5 years. The types and frequency of adverse events noted in these pediatric patients were similar to those previously documented in clinical trials with flumazenil to reverse conscious sedation in adults. No patient experienced a serious adverse event attributable to flumazenil.
The safety and efficacy of flumazenil in the reversal of conscious sedation in pediatric patients below the age of 1 year have not been established (see CLINICAL PHARMACOLOGY: Pharmacokinetics in Pediatric Patients).
The safety and efficacy of flumazenil have not been established in pediatric patients for reversal of the sedative effects of benzodiazepines used for induction of general anesthesia, for the management of overdose, or for the resuscitation of the newborn, as no well-controlled clinical studies have been performed to determine the risks, benefits and dosages to be used. However, published anecdotal reports discussing the use of flumazenil in pediatric patients for these indications have reported similar safety profiles and dosing guidelines to those described for the reversal of conscious sedation.
The risks identified in the adult population with flumazenil use also apply to pediatric patients. Therefore, consult the CONTRAINDICATIONS, WARNINGS, PRECAUTIONS, AND ADVERSE REACTIONS sections when using flumazenil in pediatric patients.
Geriatric Use:
Of the total number of subjects in clinical studies of flumazenil, 248 were 65 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
The pharmacokinetics of flumazenil have been studied in the elderly and are not significantly different from younger patients. Several studies of flumazenil in subjects over the age of 65 and one study in subjects over the age of 80 suggest that while the doses of benzodiazepine used to induce sedation should be reduced, ordinary doses of flumazenil injection may be used for reversal.
FLUMAZENIL ADVERSE REACTIONS
Serious Adverse Reactions:
Deaths have occurred in patients who received flumazenil in a variety of clinical settings. The majority of deaths occurred in patients with serious underlying disease or in patients who had ingested large amounts of non-benzodiazepine drugs (usually cyclic antidepressants), as part of an overdose.
Serious adverse events have occurred in all clinical settings, and convulsions are the most common serious adverse events reported. Flumazenil administration has been associated with the onset of convulsions in patients with severe hepatic impairment and in patients who are relying on benzodiazepine effects to control seizures, are physically dependent on benzodiazepines, or who have ingested large doses of other drugs (mixed-drug overdose) (see WARNINGS).
Two of the 446 patients who received flumazenil in controlled clinical trials for the management of a benzodiazepine overdose had cardiac dysrhythmias (1 ventricular trachycardia, 1 junctional tachycardia).
Adverse Events in Clinical Studies:
The following adverse reactions were considered to be related to flumazenil administration (both alone and for the reversal of benzodiazepine effects) and were reported in studies involving 1875 individuals who received flumazenil in controlled trials. Adverse events most frequently associated with flumazenil alone were limited to dizziness, injection site pain, increased sweating, headache, and abnormal or blurred vision (3% to 9%).
Body as a Whole: fatigue (asthenia, malaise), headache, injection site pain 1, injection site reaction (thrombophlebitis, skin abnormality, rash)
Cardiovascular System: cutaneous vasodilation ( sweating, flushing, hot flushes)
Digestive System: nausea, vomiting (11%)
Nervous System: agitation (anxiety, nervousness, dry mouth, tremor, palpitations, insomnia, dyspnea, hyperventilation)1, dizziness (vertigo, ataxia) (10%), emotional liability (crying abnormal, depersonalization, euphoria, increased tears, depression, dysphoria, paranoia)
Special Senses: abnormal vision (visual field defect, diplopia), paresthesia (sensation abnormal, hypoesthesia)
All adverse reactions occurred in 1% to 3% of cases unless otherwise marked.
Observed percentage reported if greater than 9%.
The following adverse events were observed infrequently (less than 1%) in the clinical studies, but were judged as probably related to flumazenil administration and/or reversal of benzodiazepine effects:
Nervous System: confusion (difficulty concentrating, delirium), convulsions (see WARNING), somnolence (stupor)
Special Senses: abnormal hearing (transient hearing impairment, hyperacusis, tinnitus)
The following adverse events occurred with frequencies less than 1% in the clinical trials. Their relationship to flumazenil administration is unknown, but they are included as alerting information for the physican.
Body as a whole: rigors, shivering
Cardiovascular System: arrhythmia (atrial, nodal, ventricular extrasystoles), bradycardia, tachycardia, hypertension, chest pain
Digestive System: hiccup
Nervous System: speech disorder (dysphonia, thick tongue)
Not included in this list is operative site pain that occurred with the same frequency in patients receiving placebo as in patients receiving flumazenil for reversal of sedation following a surgical procedure.
Additional Adverse Reactions Reported During Postmarketing Experience:
The following events have been reported during postapproval use of flumazenil.
Nervous System: Fear, panic attacks in patients with a history of panic disorders. Withdrawal symptoms may occur following rapid injection of flumazenil in patients with long-term exposure to benzodiazepines.
DRUG ABUSE AND DEPENDENCE
Flumazenil acts as a benzodiazepine antagonist, blocks the effects of benzodiazepines in animals and man, antagonizes benzodiazepine reinforcement in animal models, produces dysphoria in normal subjects, and has had no reported abuse in foreign marketing.
Although flumazenil injection has a benzodiazepine-like structure it does not act as a benzodiazepine agonist in man and is not a controlled substance.
OVERDOSAGE
There is limited experince of acute overdose with flumazenil.
There is no specific antidote for overdose with flumazenil. Treatment of an overdose with flumazenil should consist of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient.
Intravenous bolus administration of doses ranging from 2.5 to 100 mg (exceeding those recommended) of flumazenil, when administered to healthy normal volunteers in the absence of a benzodiazepine agonist, produced no serious adverse reactions, severe signs or symptoms, or clinically significant laboratory test abnormalities. In clinical studies, most adverse reactions to flumazenil were an extension of the pharmacologic effects of the drug in reversing benzodiazepine effects.
Reversal with an excessively high dose of flumazenil may produce anxiety, agitation, increased muscle tone, hyperesthesia and possibly convulsions. Convulsions have been treated with barbiturates, benzodiazepines and phenytoin, generally with prompt resolution of the seizures (see WARNINGS).
DOSAGE & ADMINISTRATION
Flumazenil injection is recommended for intravenous use only. It is compatible with 5% dextrose in water, lactated Ringer's and normal saline solutions. If flumazenil injection is drawn into a syringe or mixed with any of these solutions, it should be discarded after 24 hours. For optimum sterility, flumazenil injection should remain in the vial until just before use. As with all parenteral drug products, flumazenil injection should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
To minimize the likelihood of pain at the injection site, flumazenil injection should be administered through a freely running intravenous infusion into a large vein.
Reversal of Conscious Sedation:
Adult Patients:
For the reversal of sedative effects of benzodiazepines administered for conscious sedation, the recommended initial dose of flumazenil injection is 0.2 mg (2 mL) administered intravenously over 15 seconds. If the desired level of consciousness is not obtained after waiting an additional 45 seconds, a second dose of 0.2 mg (2 mL) can be injected and repeated at 60-second intervals where necessary (up to a maximum of 4 additional times) to a maximum total dose of 1 mg (10 mL). The dosage should be individualized based on the patient's response, with most patients responding to doses of 0.6 mg to 1 mg (see INDIVIDUALIZATION OF DOSAGE).
In the event of resedation, repeated doses may be administered at 20-minute intervals as needed. For repeat treatment, no more than 1 mg (given as 0.2 mg/min) should be administered at any one time, and no more than 3 mg should be given in any one hour.
It is recommended that flumazenil injection be administered as the series of small injections described (not as a single bolus injection) to allow the practitioner to control the reversal of sedation to the approximate endpoint desired and to minimize the possiblility of adverse effects (see INDIVIDUALIZATION OF DOSAGE).
Pediatric Patients:
For the reversal of the sedative effects of benzodiazepines administered for conscious sedation in pediatric patients greater than 1 year of age, the recommended initial dose is 0.01 mg/kg (up to 0.2 mg) administered intravenously over 15 seconds. If the desired level of consciousness is not obtained after waiting an additional 45 seconds, further injections of 0.01 mg/kg (up to 0.2 mg) can be administered and repeated at 60-second intervals where necessary (up to a maximum of 4 additional times) to a maximum total dose of 0.05 mg/kg or 1mg, whichever is lower. The dose should be individualized based on the patient's response. The mean total dose administered in the pediatric clinical trial of flumazenil was 0.65 mg (range: 0.08 mg to 1.00 mg). Approximately one-half of patients required the maximum of five injections.
Resedation occurred in 7 of 60 pediatric patients who were fully alert 10 minutes after the start of flumazenil administration (see PRECAUTIONS: Pediatric Use). The safety and efficacy of repeated flumazenil administration in pediatric patients experiencing resedation have not been established.
It is recommended that flumazenil injection be administered as the series of small injections described (not as a single bolus injection) to allow the practitioner to control the reversal of sedation to the approximate endpoint desired and to minimize the possibility of adverse effects (see INDIVIDUALIZATION OF DOSAGE).
The safety and efficacy of flumazenil in the reversal of conscious sedation in pediatric patients below the age of 1 year have not been established.
Reversal of General Anesthesia in Adult Patients:
For the reversal of the sedative effects of benzodiazepines administered for general anesthesia, the recommended initial dose of flumazenil injection is 0.2 mg (2 mL) administered intravenously over 15 seconds. If the desired level of consciousness is not obtained after waiting an additional 45 seconds, a further dose of 0.2 mg (2 mL) can be injected and repeated at 60-second intervals where necessary (up to a maximum of 4 additional times) to a maximum total dose of 1 mg (10 mL). The dosage should be individualized based on the patient's response, with most patients responding to doses of 0.6 mg to 1 mg (see INDIVIDUALIZATION OF DOSAGE).
In the event of resedation, repeated doses may be administered at 20-minute intervals as needed. For repeat treatment, no more than 1 mg (given as 0.2 mg/min) should be administered at any one time, and no more than 3 mg should be given in any one hour.
It is recommended that flumazenil injection be administered as the series of small injections described (not as a single bolus injection) to allow the practitioner to control the reversal of sedation to the approximate endpoint desired and to minimize the possibility of adverse effects (see INDIVIDUALIZATION OF DOSAGE).
Management of Suspected Benzodiazepine Overdose in Adult Patients:
For initial management of a known or suspected benzodiazepine overdose, the recommended initial dose of flumazenil injection is 0.2 mg (2 mL) administered intravenously over 30 seconds. If the desired level of consciousness is not obtained after waiting 30 seconds, a further dose of 0.3 mg (3 mL) can be administered over another 30 seconds at 1-minute intervals up to a cumulative dose of 3 mg.
Do not rush the administration of flumazenil injection. Patients should have a secure airway and intravenous access before administration of the drug and be awakened gradually (see PRECAUTIONS).
Most patients with a benzodiazepine overdose will respond to a cumulative dose of 1 mg to 3 mg of flumazenil injection, and doses beyond 3 mg do not reliably produce additional effects. On rare occasions, patients with a partial response at 3 mg may require additional titration up to a total dose of 5 mg (administered slowly in the same manner).
If a patient has not responded 5 minutes after receiving a cumulative dose of 5 mg of flumazenil injection, the major cause of sedation is likely not to be due to benzodiazepines, and additional flumazenil injection is likely to have no effect.
In the event of resedation, repeated doses may be given at 20-minute intervals if needed. For repeat treatment, no more than 1 mg (given as 0.5 mg/min) should be given at any one time and no more than 3 mg should be given in any one hour.
Safety and Handling:
Flumazenil injection is supplied in sealed dosage forms and poses no known risk to the healthcare provider. Routine care should be taken to avoid aerosol generation when perparing syringes for injection, and spilled medication should be rinsed from the skin with cool water.
HOW SUPPLIED
Flumazenil injection contains 0.1 mg of flumazenil per mL and is supplied as follows:
NDC 0781-3003-92 multiple-dose vials of 5 mL in boxes of 10.
NDC 0781-3003-95 multiple-dose vials of 10 mL in boxes of 10.
Store at 20-25 degree Celsius (68-77 degree Fahrenheit) (see USP Controlled Room Temperature).
PACKAGE LABEL


FlumazenilFlumazenil INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||