Fluorecein sodium and Proparacaine Hydrochloride
Fluorecein sodium and Proparacaine Hydrochloride Opthahalmic Solution, USP
FULL PRESCRIBING INFORMATION
DESCRIPTION:
Fluorescein Sodium and Proparacaine Hydrochloride Ophthalmic Solution, USP (Sterile) is a sterile ophthalmic solution combining the disclosing action of Fluorescein with the anesthetic action of Proparacaine Hydrochloride.
The active ingredient, Fluorescein Sodium, has the chemical name Spiro [isobenzofuran-1 (3H), 9'-[9H]xanthene]-3-one, 3' ,6' dihydroxy-,disodium salt. It is represented by the following structural formula:
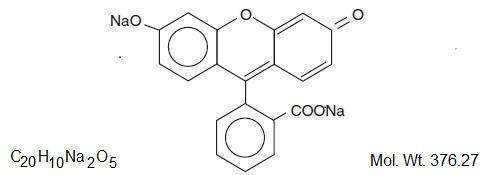
The active ingredient, Proparacaine Hydrochloride, has the chemical name Benzoic acid, 3-amino-4-propoxy-, 2-[diethylamino]ethyl ester monohydrochloride. It is represented by the following structural formula:
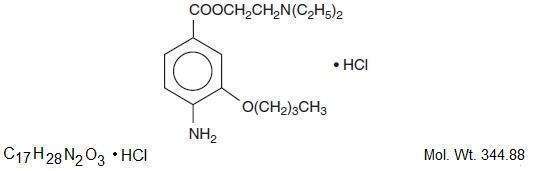
EACH mL CONTAINS: ACTIVES: Fluorescein Sodium, USP, 0.25% [2.5 mg]. Proparacaine Hydrochloride, USP, 0.5% [5mg]; INACTIVES: Povidone, Boric Acid, Water for Injection, Sodium Hydroxide, or/and Hydrochloric Acid may be added to adjust pH.
PRESERVATIVE: Methylparaben 0.1%.
CLINICAL PHARMACOLOGY
This product is the combination of a disclosing agent with a rapidly acting anesthetic of short duration.
For procedures requiring a disclosing agent in combination with an anesthetic agent such as tonometry, gonioscopy, removal of corneal foreign bodies and other short corneal or conjunctival procedures.
CONTRAINDICATIONS:
Known hypersensitivity to any component of this product.
WARNINGS:
Avoid contamination - do not touch tip of sterile dropper used to dispense solution to any surface. Replace container closure immediately after using. Prolonged use of a topical ocular anesthetic is not recommended. It may product permanent corneal opacification with accompanying visual loss.
PRECAUTIONS:
This product should be used cautiously and sparingly in patients with known allergies, cardiac disease, or hyperthyroidism. The long-term toxicity is unknown; prolonged use may possibly delay wound healing. Although exceedingly rare with ophthalmic application of local anesthetics, it should be borne in mind that systemic toxicity manifested by central nervous system stimulation followed by depression may occur. Protection of the eye from irritating chemicals, foreign bodies and rubbing during the period of anesthesia is very important. Tonometers soaked in sterilizing or detergent solutions should be thoroughly rinsed with sterile distilled water prior to use. Patients should be advised to avoid touching the eye until the anesthesia has worn off.
ADVERSE REACTIONS:
Occasional temporary stinging, burning, and conjunctival redness have been reported after use of ocular anesthetics, as well as a rare, severe, immediate-type, apparent hyper-allergic corneal reaction, with acute, intense and diffuse epithelial keratitis, a gray, ground glass appearance, sloughing of large areas of necrotic epithelium, corneal filaments and sometimes, iritis with descementitis. Allergic contact dermatitis with drying and fissuring of the fingertips has been reported.
To report SUSPECTED ADVERSE REACTIONS, contact Altaire Pharmaceuticals, Inc. at (631) 722-5988.
DOSAGE AND ADMINISTRATION:
Usual Dosage: Removal of foreign bodies and sutures, and for tonometry, 1 to 2 drops (in single instillations) in each eye before operating.
Deep Ophthalmic Anesthesia: 1 drop in each eye every 5 to 10 minutes for 5-7 doses.
NOTE: The use of an eye patch is recommended.
STORAGE:
Store in refrigerator at 2°-8°C (36°-46°F). Can be stored at room temperature for up to 1 month.
HOW SUPPLIED:
Fluorescein Sodium and
Proparacaine Hydrochloride Ophthalmic Solution, USP (Sterile) is
supplied in a glass or plastic bottle with a controlled tip applicator
in the following sizes:
5 mL fill (glass bottle) with dropper

_dae037ad.jpg)

DO NOT USE IS IMPRINTED SEAL ON CAP IS BROKEN OR MISSING.
KEEP OUT OF REACH OF CHILDREN
FOR OPHTHALMIC USE ONLY
Manufactured By:
Altaire Phamacueticals, Inc.
311 West Lane
Aquebogue, NY 119311
Tel: 631-722-5988
Fluorecein sodium and Proparacaine HydrochlorideFluorecein sodium and Proparacaine Hydrochloride Opthahalmic Solution SOLUTION/ DROPS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||