Fluorescein Sodium and Benoxinate Hydrochloride
Fluorescein Sodium and Benoxinate Hydrochloride Ophthalmic Solution USP, 0.25%/0.4% (Sterile)Rx onlyFOR USE IN THE EYES ONLY
FULL PRESCRIBING INFORMATION: CONTENTS*
- FLUORESCEIN SODIUM AND BENOXINATE HYDROCHLORIDE DESCRIPTION:
- CLINICAL PHARMACOLOGY:
- FLUORESCEIN SODIUM AND BENOXINATE HYDROCHLORIDE INDICATIONS AND USAGE:
- FLUORESCEIN SODIUM AND BENOXINATE HYDROCHLORIDE CONTRAINDICATIONS:
- WARNINGS: NOT FOR INJECTION- FOR TOPICAL OPHTHALMIC USE ONLY
- PRECAUTIONS:
- FLUORESCEIN SODIUM AND BENOXINATE HYDROCHLORIDE ADVERSE REACTIONS:
- FLUORESCEIN SODIUM AND BENOXINATE HYDROCHLORIDE DOSAGE AND ADMINISTRATION:
- HOW SUPPLIED:
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
DESCRIPTION:
Fluorescein Sodium and Benoxinate Hydrochloride Ophthalmic Solution USP, 0.25%/0.4% is a disclosing agent with rapid anesthetic action and short duration.
Fluorescein sodium is represented by the following structural formula:
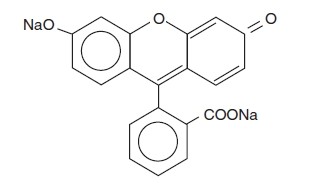
C20H10Na2O5
Mol. Wt. 376.28
Chemical Name: Spiro (isobenzofuran-1 (3H),9'-(9H) xanthene)-3-one, 3',6' dihydroxy-, disodium salt.
Benoxinate hydrochloride is represented by the following structural formula:
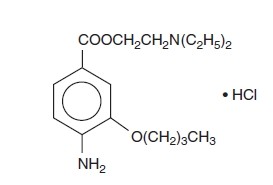
C17H28N2O3 • HCl
Mol. Wt. 344.88
Chemical Name: 2-(Diethylamino) ethyl 4-amino-3-butoxybenzoate monohydrochloride.
Each mL Contains: ACTIVES: Fluorescein Sodium 2.5 mg (0.25%), Benoxinate Hydrochloride 4 mg (0.4%); INACTIVES: Povidone, Boric Acid, Purified Water. Hydrochloric Acid may be added to adjust pH (4.3 – 5.3). PRESERVATIVE ADDED: Chlorobutanol 1%.
CLINICAL PHARMACOLOGY:
Fluorescein Sodium and Benoxinate Hydrochloride Ophthalmic Solution USP, 0.25%/0.4% is the combination of a disclosing agent with a rapidly acting anesthetic of short duration.
INDICATIONS AND USAGE:
For procedures requiring a disclosing agent in combination with a topical ophthalmic anesthetic agent such as tonometry, gonioscopy, removal of corneal foreign bodies and other short corneal or conjunctival procedures.
CONTRAINDICATIONS:
Known hypersensitivity to any component of this product.
WARNINGS: NOT FOR INJECTION- FOR TOPICAL OPHTHALMIC USE ONLY
Prolonged use of a topical ocular anesthetic is not recommended. It may produce permanent corneal opacification with accompanying visual loss.
PRECAUTIONS:
Fluorescein Sodium and Benoxinate Hydrochloride Ophthalmic Solution USP, 0.25%/0.4% should be used cautiously and sparingly in patients with known allergies, cardiac disease, or hyperthyroidism. The long-term toxicity is unknown; prolonged use may possibly delay wound healing. Although exceedingly rare with ophthalmic application of local anesthetics, it should be borne in mind that systemic toxicity manifested by central nervous system stimulation followed by depression may occur. Protection of the eye from irritation chemicals, foreign bodies and rubbing during the period of anesthesia is very important. Tonometers soaked in sterilizing or detergent solutions should be thoroughly rinsed with sterile distilled water prior to use. Patients should be advised to avoid touching the eye until the anesthesia has worn off.
Pregnancy:
Pregnancy Category C. Animal reproduction studies have not been conducted with Fluorescein Sodium and Benoxinate Hydrochloride Ophthalmic Solution USP, 0.25%/0.4%. It is also not known whether Fluorescein Sodium and Benoxinate Hydrochloride Ophthalmic Solution USP, 0.25%/0.4% can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Fluorescein Sodium and Benoxinate Hydrochloride Ophthalmic Solution USP, 0.25%/0.4% should be given to a pregnant woman only if clearly needed.
Nursing Mothers:
Caution should be exercised when Fluorescein Sodium and Benoxinate Hydrochloride Ophthalmic Solution USP, 0.25%/0.4% is administered to a nursing woman.
Pediatric Use:
Safety and effectiveness in pediatric patients have not been established.
ADVERSE REACTIONS:
Occasional temporary stinging, burning, and conjunctival redness have been reported after use of ocular anesthetics, as well as a rare, severe, immediate-type, apparent hyper-allergic corneal reaction, with acute, intense and diffuse epithelial keratitis, a gray, ground glass appearance, sloughing of large areas of necrotic epithelium, corneal filaments and sometimes, iritis with descemetitis.
Allergic contact dermatitis with drying and fissuring of the fingertips has been reported
DOSAGE AND ADMINISTRATION:
Usual Dosage: Removal of foreign bodies and sutures, and for tonometry, 1 to 2 drops (in single instillations) in each eye before operating.
HOW SUPPLIED:
Fluorescein Sodium and Benoxinate Hydrochloride Ophthalmic Solution USP, 0.25%/0.4% is supplied in a glass bottle with a sterilized dropper in the following size:
5 mL - Prod. No. 30107
Storage:
Store in a refrigerator at 2°-8°C (36°-46°F).
User may store at room temperature up to one month.
Keep tightly closed.

KEEP OUT OF REACH OF CHILDREN.
Revised January 2008
Bausch & Lomb Incorporated
Tampa, FL 33637
©Bausch & Lomb Incorporated
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
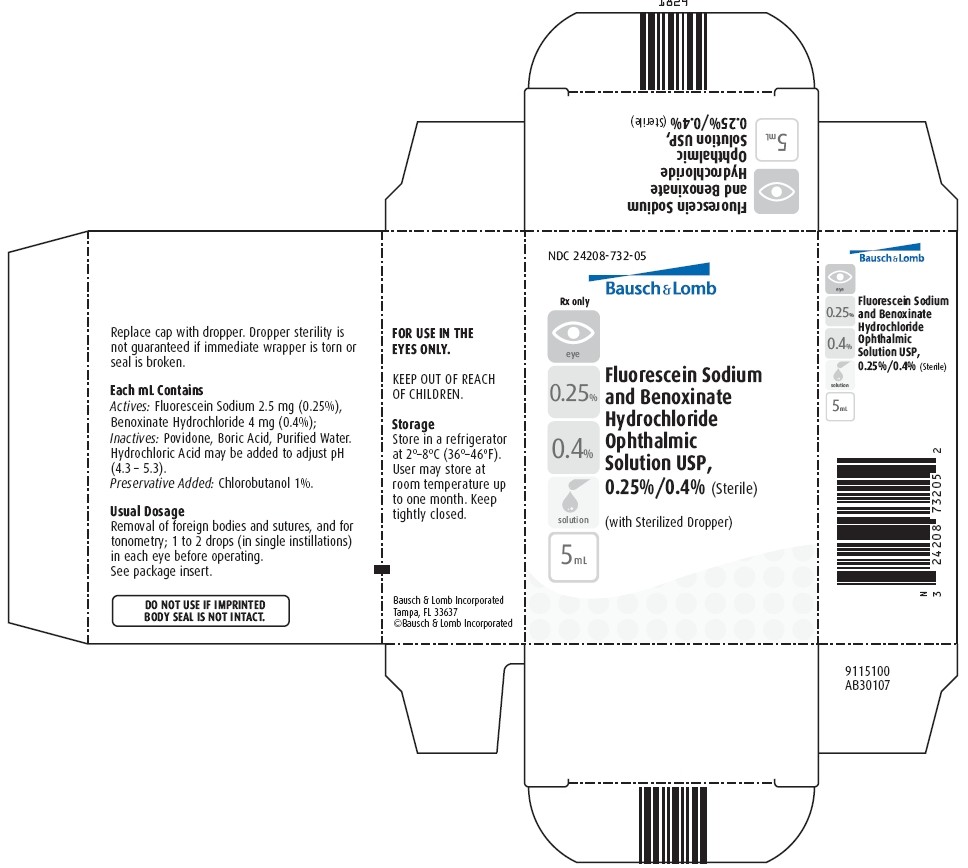
NDC 24208-732-05
Bausch & Lomb
Rx only
Fluorescein Sodium and Benoxinate Hydrochloride Ophthalmic Solution USP, 0.25%/0.4% (Sterile)
(with Sterilized Dropper)
[icon-eye] [icon-0.25%] [icon-0.4%] [icon-solution] [icon-5mL]
Fluorescein Sodium and Benoxinate HydrochlorideFluorescein Sodium and Benoxinate Hydrochloride SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||