Fluoride
Prescribing Information
FULL PRESCRIBING INFORMATION: CONTENTS*
- FLUORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- FLUORIDE INDICATIONS AND USAGE
- FLUORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- FLUORIDE ADVERSE REACTIONS
- OVERDOSAGE
- FLUORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- STORAGE
- Inactive Ingredients
- Base Label
- Inside Label
- Outside Label
FULL PRESCRIBING INFORMATION
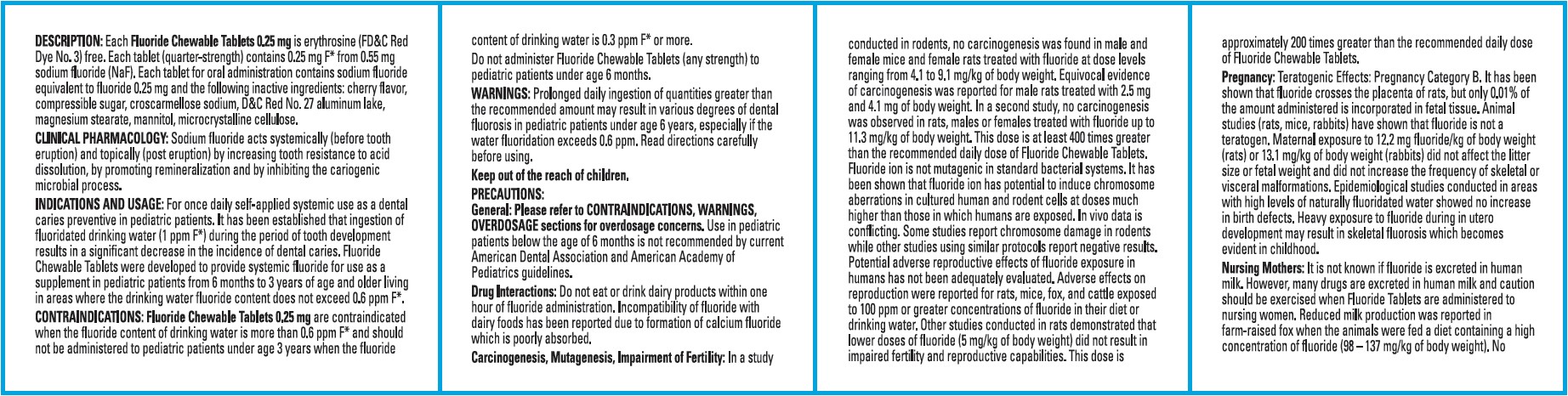
FLUORIDE DESCRIPTION
Each Fluoride Chewable Tablet 0.25 mg is erythrosine (FD&C Red Dye No.3) free. Each tablet 0.25 mg (quarter strength) contains 0.25 mg F* from 0.55 mg sodium fluoride (NaF)
CLINICAL PHARMACOLOGY
Sodium fluoride acts systemically (before tooth eruption) and topically (post eruption) by increasing tooth resistance to acid dissolution, by promoting remineralization and by inhibiting the cariogenic microbial process.
FLUORIDE INDICATIONS AND USAGE
For once daily self-applied systemic use as a dental caries preventive in pediatric patients. It has been established that ingestion of fluoridated drinking water (1 ppm F*) during the period of tooth development results in a significant decrease in the incidence of dental caries. Fluoride Chewable Tablets were developed to provide systemic fluoride for use as a supplement in pediatric patients from 6 months to 3 years of age and older living in areas where the drinking water fluoride content does not exceed 0.6 ppm F*.
FLUORIDE CONTRAINDICATIONS
Fluoride Chewable Tablets 0.25 mg are contraindicated when the fluoride content of drinking water is more than 0.6 ppm F* and should not be administered to pediatric patients under age 3 years when the fluoride content of drinking water is 0.3 ppm F* or more.
Do not administer Fluoride Chewable Tablets (any strength) to pediatric patients under age 6 months.
WARNINGS
Prolonged daily ingestion of quantities greater than the recommended amount may result in various degrees of dental fluorosis in pediatric patients under age 6 years, especially if the water fluoridation exceeds 0.6 ppm. Read directions carefully before using.
Keep out of the reach of children.
PRECAUTIONS
GeneralCONTRAINDICATIONS, WARNINGSOVERDOSAGE
Drug Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
In vivoPregnancy
Nursing Mothers
Pediatric Use
GERIATRIC USE
FLUORIDE ADVERSE REACTIONS
Allergic rash and other idiosyncrasies have rarely been reported.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
OVERDOSAGE
FLUORIDE DOSAGE AND ADMINISTRATION
Dissolve in the mouth or chew before swallowing, preferably at bedtime after brushing teeth.
HOW SUPPLIED
Chewable tablets containing 0.25 mg fluoride are pink-colored, cherry flavor, un-scored, round, debossed "104". Available in bottles of 120's.
STORAGE
Store at Controlled Room Temperature, 20°-25°C (68°-77°F). [See USP Controlled Room Temperature].
*F from sodium fluoride.
Inactive Ingredients
Base Label

Inside Label
Outside Label

FluorideSodium Fluoride TABLET, CHEWABLE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||