Folic Acid
NCS HealthCare of KY, Inc dba Vangard Labs
Folic Acid Tabs
FULL PRESCRIBING INFORMATION: CONTENTS*
- FOLIC ACID DESCRIPTION
- CLINICAL PHARMACOLOGY
- FOLIC ACID INDICATIONS AND USAGE
- FOLIC ACID CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- FOLIC ACID ADVERSE REACTIONS
- OVERDOSAGE
- FOLIC ACID DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
FOLIC ACID DESCRIPTION
Folic Acid,N-[p-[[(2-Amino-4-hydroxy-6-pteridiny)methyl]amino]benzoyl]-L-glutamic acid, is a B complex vitamin containing a pteridine moiety linked by a methylene bridge to para-aminobenzoic acid, which is joined by a peptide linkage to glutamic acid. Conjugates of folic acid are present in a wide vaiety of foods, particularly liver, kidneys, yeast, and leafy green vegetables. Commercially available folic acid is prepared synthetically. Folic acid occurs as a yellow or yellowish-orange crystalline powder and is very slightly soluble in water and insoluble in alcohol. Folic acid is readily soluble in dilute solutions of alkali hydroxides and carbonates,and solutions of the drug may be prepared with the aid of sodium hydroxide ore sodium salt of folic acid (sodium folate). Aqueous solutions of folic acid are heat sensitive and rapidly decompose in the presence of light and/or riboflavin; solutions should be stored in a cool dry place protected from light.
The structural formula is as follows:
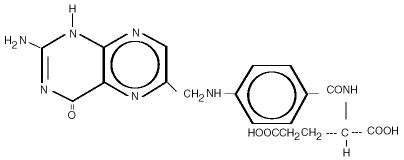
The molecular formula is C19H19N7O6, and the molecular wieght is 441.40.
Each tablet for oral administration contains folic acid 1mg and the following inactive ingredients: colloidal silicon dioxide, lactose monohydrate, microcrystalline cellulose, sodium starch glycolate and stearic acid.
CLINICAL PHARMACOLOGY
Folic Acid acts on megaloblastic bone marrow to produce a normoblastic marrow.
In man, an exogenous source of folate is required for nucleoprotein synthesis and the maintenance of normal erythropoiesis. Folic Acid is the precursor of tetrahydrofolic acid, which is involved as a cofactor for transformylation reactions in the biosynthesis of purines and thymidylates of nucleic acids. Impairment of thymidylate synthesis in patients with folic acid defeciency is thought to account for the defective deoxyribonucleic acid (DNA) synthesis that leads to megaloblast formation and megaloblastic and macrocytic anemias.
Folic acid is absorbed rapidly from the small intestine, primarily from the proximal portion. Naturally occurring conjugated folates are reduced enzymatically to folic acid in the gastointestinal tract prior to absorption. Folic acid appears in the plasma approximately 15 to 30 minutes after an oral dose; peak levels are generally reached within 1 hour. After intravenous administration, the drug is rapidly cleared from the plasma. Cerebrospinal fluid levels of folic acid are several times greater than serum levels of the drug; Folic acid is metabolized in the liver to 7, 8 -dihydrofolic acid and eventually to 5, 6, 7, 8-tetrahydrofolic acid with the aid of reduced diphosphopyridine nucleotide (DPNH) and folate reductases. Tetrahydrofolic acid is linked in the N5 or N10 positions with formyl, hydroxymethyl, methyl, or formimino groups. N5-formyltetrahydrofolic acid is leucovorin. Tetrahydrofolic acid derivatives are distributed to all body tissues but are stored primarily in the liver. Normal serum levels of total folate have been reported to be 5 to 15 ng/mL; normal cerebrospinal fluid levels are approximately 16 to 21 ng/mL. Normal erythrocyte folate levels have been reported to range from 175 to 316 ng/mL. In general, folate serum levels below 5ng/mL indicate folate deficiency, and levels below 2ng/mL usually result in megaloblastic anemia.
After a single oral dose fo 100 mcg of folic acid in a limited number of normal adults, only a trace amount of the drug appeared in the urine. An oral dose of 5 mg in 1 study and a dose of 40mcg/kg of body weight in another study resulted in approximately 50% of the dose appearing in the urine. After a single oral dose of 15 mg, up to 90 % of the dose was recovered in the urine. A majority of the metabolic products appeared in the urine after 6 hours; excretion was generally complete within 24 hours. Small amounts of orally administered folic acid have also been recovered in the feces. Folic acid is also excreted in milk of lactating mothers.
FOLIC ACID INDICATIONS AND USAGE
Folic acid is effective in the treatment of megaloblastic anemias due to a deficiency of folic acid (as may be seen in tropical or nontropical spure) and in anemias of nutritional origin, pregnancy, infancy, or childhood.
FOLIC ACID CONTRAINDICATIONS
Folic acid is contraindicated in patients who have shown previous intolerance to the drug.
WARNINGS
Administration of folic acid alone is improper therapy for pernicious anemia and other megaloblastic anemias in which vitamin B12 is deficient.
PRECAUTIONS
General
Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurologic manufestations remain progrssive.
There os a potential danger in administering folic acid to patients with undiagnosed anemia, since folic acid may abscure the idagnosis of pernicious anemia by alleviating the hematologic manifestations of the disease while allowing the neurologic complications to progress. This may result in severe nervous system damage before the correct diagnosis is made. Adequate doses of vitamin B12 may prevent, halt, or improve the neurologic changes caused by pernicious anemia.
Drug Interactions
There is evidence that the anticonvulsant action of phenytoin is antagonized by folci acid. A patient whose apilepsy is completely controlled by phenytoin may require increased doses to prevent convulsions if folic acid is given.
Folate deficiency may result from increased loss of folate, as in renal dialysis and/or interference with metabolism (i.g., folic acid antagonists such as methotrxate); the administration of anticonvulsants, such as diphenythdantoin, primidone, and barbiturates; alcohol consumption and , especially, alcoholic cirrhosis; and the administration of pyrimethamine and nitrofurantoin.
False low serum and red cell folate levels may occur if the patient has been taking antibiotics, such as tetracycline, which suppress the growth of Lactobacillus casel.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evalluate carcinogenic potential and studies to evaluate the mutagenic potential or effect on fertitlity have not been conducted.
Pregnancy
Pregnancy - Teratogenic Effects - Pregnancy Category A. Folic acid is usually indicated in the treatment of megaloblastic anemias of pregnancy. Folic acid requirements are markedly increased during pregnancy, and deficiency will result in fetal damage. (See INDICATIONS AND USAGE).
Studies in pregnant women have not shown that folic acid increases the risk of fetal abnormalities if administered during pregnancy. If the drug is used during pregnancy, the possibility of fetal harm appears remote. Because studies cannot rule out the possibility of harm, however, folic acid should be used during pregnancy only if clearly needed.
Nursing Mothers
Folic acid is excreted in the milk of lactating mothers. During lactation, folic acid requirements are markedly increased; however, amounts present in human milk are adequate to fulfill infant requirements, although supplementation may be needed in low-birth-wieht infants, in those who are breast-fed by mothers with folic acid deficiency (50 mcg daily), or in those with infections or prolonged diarrhea.
FOLIC ACID ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parental administration of folic acid.
Folic acid is relatively nontoxic in man. Rare instances of allergic responses to folic acid preparations have been reported and have included erythema, skin rash, itching, general malaise, and respiratory difficulty due to bronchospasm. One patient experienced symproms suggesting anaphylaxis following injection of the drug. Gastrointestinal side effects, including anorexia, nausea, abdominal distention, flatullence, and a bitter or bad taste, have been reported in patients receiving 15 mg of folic acid daily for 1 month. Other side effects reported in patients receiving 15 mg daily include altered sleep patterns, difficulty in concentrating, irritability, over-activity, excitement, mental depression, confusion, and impaired judgment. Decreased vitamin B12 serum levels may occur in patients receiving prolonged folic acid therapy
In an uncontrolled study, orally administered folic acid was reported to increase the incidence of seizures in some epileptic patients receiving phenobarbital, primidone, or diphenythdantoin. Another investigator reported decreased diphenythydantoin serum levels in folate-deficient patients receiving diphenythydantoin who were treated with 5mg or 15mg of folic acid daily.
To report SUSPECTED ADVERSE REACTIONS, contact West-ward Pharmaceutical Corp. at 1-877-233-2001, or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
Except during pregnancy and lactation, folic acid should not be given in therapeutic doses greater than 0.4mg daily until pernicious anemia has been ruled out. Patients with pernicious anemia receiving more than 0.4mg of folic acid daily who are inadequately treated with Vitamin B12 may show reversion of the hematologic parameters to normal, but neurologic manifestations due to vitamin B12 deficiency will progress. Doses of folic acid exceeding the Recommended Dietary Allowance (RDA) should not be included in multivitamin preparations; if therapeutic amounts are necessary, folic acid should be given separately.
FOLIC ACID DOSAGE AND ADMINISTRATION
Oral administration is preferred. Although most patients with malabsorption cannot absorb food folates, they are able to absorb folic acid given orally. Parneteral administration is not advocated but may be necessary in some individuals (e.g., patients receiving parenteral or enteral alimentation). Doses greater than 0.1mg should not be used unless anemia due to vitamin B12 deficiency has been ruled out or is being adequately treated with cobalamin. Daily doses greater than 1mg do not enhance the hematologic effect, and most of the excess is excreted unchanged in the urine.
The usual therapeutic dosage in adults and children (regardless of age) is up to 1 mg daily. Resistant cases may require larger doses.
When clinical symptoms have subsided and the blood picture has become normal, a daily maintenance level should be used, i.e., 0.1mg for infants and up to 0.3mg for children under 4 years of age, 0.4mg for adults and children 4 or more years of age, and 0.8mg for pregnant and lactating women, but never less than 0.1mg/day. Patients should be kept under close supervision and adustment of maintenance level made if relapse appears imminent.
In the presence of alcoholism, hemolytic anemia, anticonvulsant therapy, or chronic infection, the maintenance level may need to be increased.
HOW SUPPLIED
Folic Acid Tablets USP, 1 mg are Light Yellow, Round, biconvex tablets debossed "I" on the left side of the bisect and "G" on the right side of bisect on one side and "210" on other; supplied in blistercards of 30 (NDC 0615-0664-39), 31(NDC 0615-0664-31), and 15 (NDC 0615-0664-05).
Store at 20-25 degrees C (68-77 degrees F) [See USP Controlled Room Temperature]. Protect from light and moisture.
Dispense in a tight, light-resistent container as defined in the USP using a child-resistant closure.
REFERENCES
Manufactured by:
InvaGen Pharmaceuticals, Inc.
Hauppauge, NY 11788
Distributed by:
West-Ward Pharmacuetical Corp.
Eaton town, NJ 07724
PRINCIPAL DISPLAY PANEL
Folic Acid Tabs,
USP 1mg

Folic AcidFolic Acid TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||