Furosemide
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- FUROSEMIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- FUROSEMIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- FUROSEMIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
FUROSEMIDE DESCRIPTION
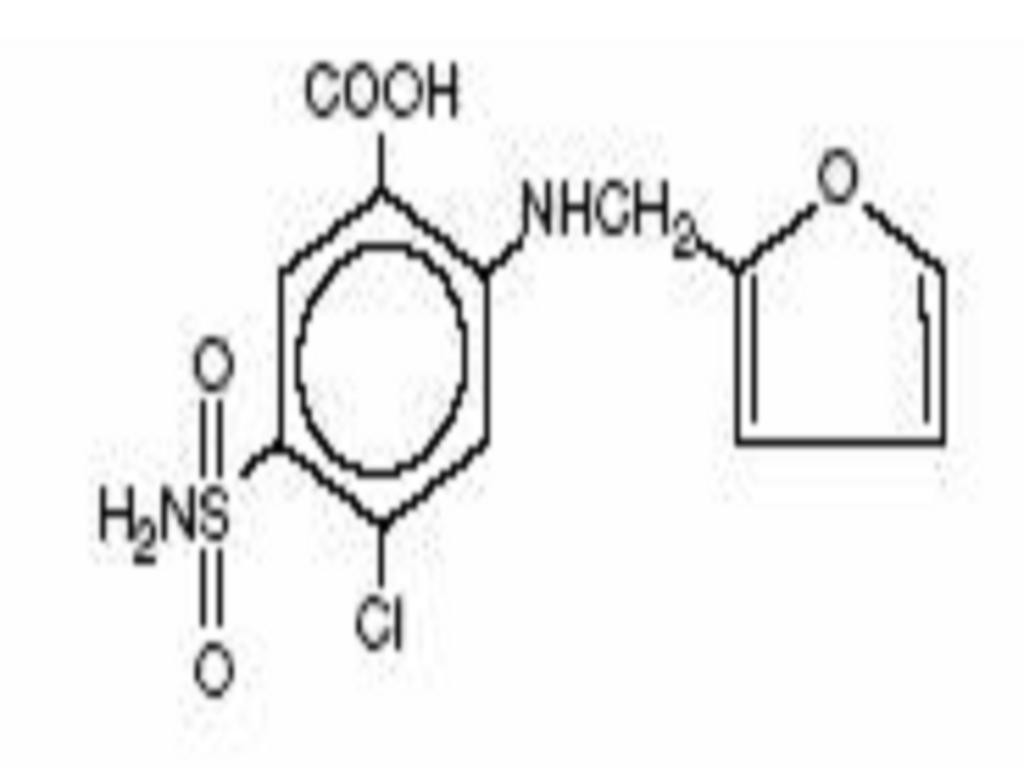
CLINICAL PHARMACOLOGY
Geriatric Population
PRECAUTIONS:Geriatric Use
INDICATIONS & USAGE
EdemaHypertension
FUROSEMIDE CONTRAINDICATIONS
WARNINGS
Drug Interactions
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
LABORATORY TESTS
Pediatric Use
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
PRECAUTIONS: GeneralDOSAGE AND ADMINISTRATION
FUROSEMIDE ADVERSE REACTIONS
Gastrointestinal System Reactions
Systemic Hypersensitivity Reactions
Central Nervous System Reactions
Hematologic Reactions
Dermatologic-Hypersensitivity Reactions
Cardiovascular Reaction
Other Reactions
OVERDOSAGE
DOSAGE & ADMINISTRATION
EdemaPRECAUTIONS: Laboratory Tests.
PRECAUTIONS: Geriatric Use
Hypertension
PRECAUTIONS: Geriatric Use
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

FurosemideFurosemide TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!