Gabapentin
FULL PRESCRIBING INFORMATION: CONTENTS*
- GABAPENTIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- CLINICAL STUDIES
- INDICATIONS & USAGE
- GABAPENTIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LABORATORY TESTS
- DRUG INTERACTIONS
- LABORATORY TESTS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- GABAPENTIN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
GABAPENTIN DESCRIPTION
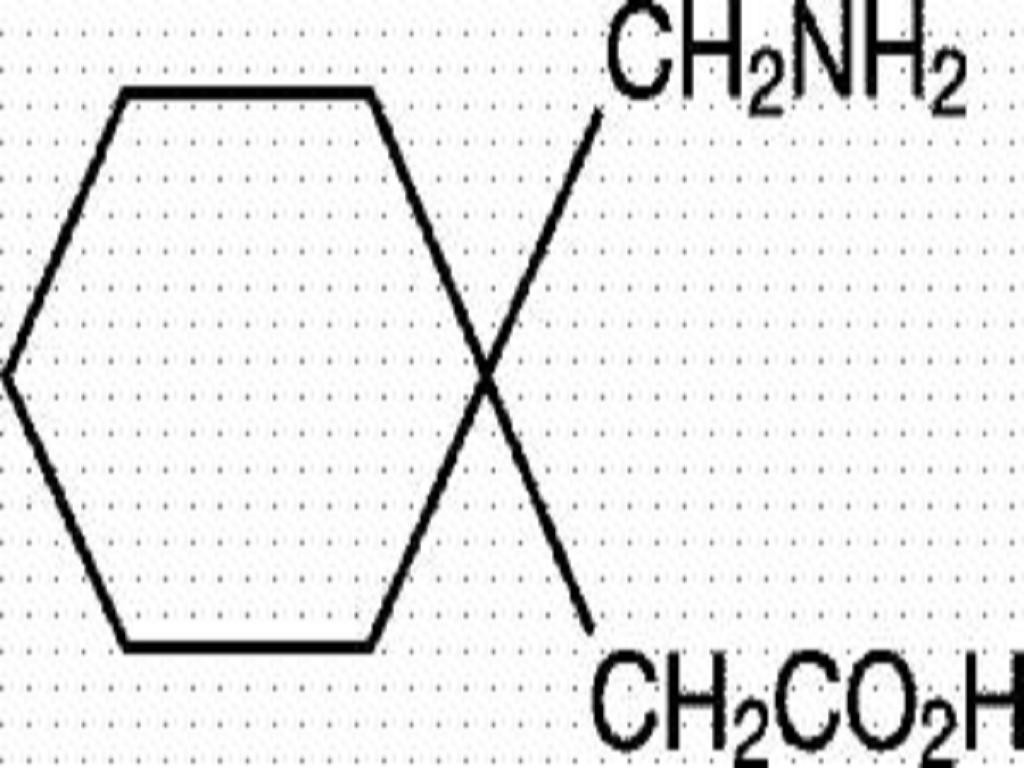
CLINICAL PHARMACOLOGY
PHARMACOKINETICS
DOSAGE AND ADMINISTRATION, Table 5
DOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION
PRECAUTIONS, Geriatric UseDOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION
CLINICAL STUDIES
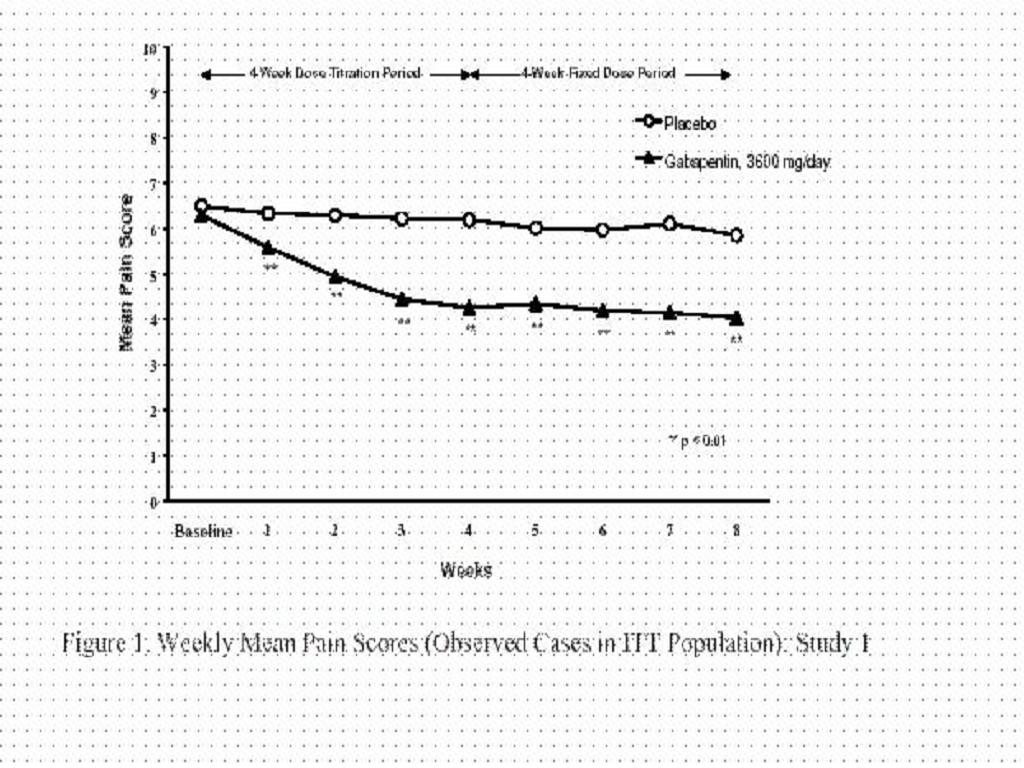
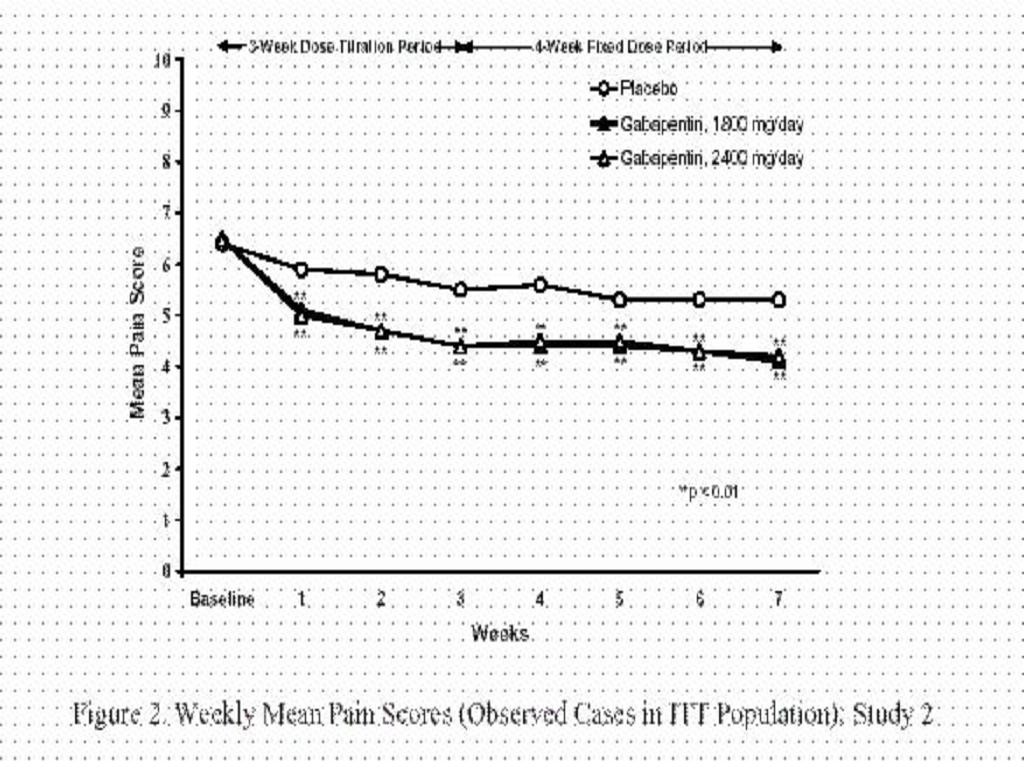


INDICATIONS & USAGE
GABAPENTIN CONTRAINDICATIONS
WARNINGS
WARNINGSPRECAUTIONS: Carcinogenesis, Mutagenesis, Impairment of Fertility
PRECAUTIONS
Information for PatientsDrug Interactions
LABORATORY TESTS
Laboratory TestsDRUG INTERACTIONS
Drug InteractionsPRECAUTIONS
LABORATORY TESTS
Drug/Laboratory Test InteractionsCARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis, Impairment of FertilityPREGNANCY
NURSING MOTHERS
Use In Nursing MothersPEDIATRIC USE
GERIATRIC USE
CLINICAL PHARMACOLOGYADVERSE REACTIONSDOSAGE AND ADMINISTRATION
GABAPENTIN ADVERSE REACTIONS
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
OVERDOSAGEDOSAGE & ADMINISTRATION
DOSAGE & ADMINISTRATIONINACTIVE INGREDIENT
INACTIVE INGREDIENTS:FERROSOFERRIC OXIDE
STARCH, CORN
D&C YELLOW NO. 10
FD&C BLUE NO. 2
FD&C RED NO. 40
GELATIN
MANNITOL
SHELLAC
PROPYLENE GLYCOL
FERRIC OXIDE RED
SILICON DIOXIDE
SODIUM LAURYL SULFATE
FERROSOFERRIC OXIDE
TALC
TITANIUM DIOXIDE
FERRIC OXIDE YELLOW
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


GabapentinGabapentin CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!