Gabapentin
Gabapentin Tablets, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- GABAPENTIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- GABAPENTIN INDICATIONS AND USAGE
- GABAPENTIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- GABAPENTIN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- GABAPENTIN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- MEDICATION GUIDE
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 600 mg (100 Tablet Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 800 mg (100 Tablet Bottle)
FULL PRESCRIBING INFORMATION
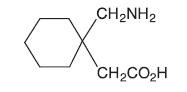
GABAPENTIN DESCRIPTION
9172
a1a2
CLINICAL PHARMACOLOGY
Mechanism of Action
AB
In vitro
Pharmacokinetics and Drug Metabolism
max
min
Elimination
Special Populations: Patients With Renal Insufficiency ,
DOSAGE AND ADMINISTRATION
Special Populations
Adult Patients With Renal Insufficiency
DOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION
PRECAUTIONS, Geriatric Use DOSAGE AND ADMINISTRATION
Pediatric
DOSAGE AND ADMINISTRATION
Clinical Studies
| Study | Study Duration |
Gabapentin (mg/day)a Target Dose |
Patients Receiving Gabapentin |
Patients Receiving Placebo |
|---|---|---|---|---|
| a Given in 3 divided doses (TID) |
||||
| 1 |
8 weeks |
3600 |
113 |
116 |
| 2 |
7 weeks |
1800, 2400 |
223 |
111 |
| Total |
336 |
227 |
||
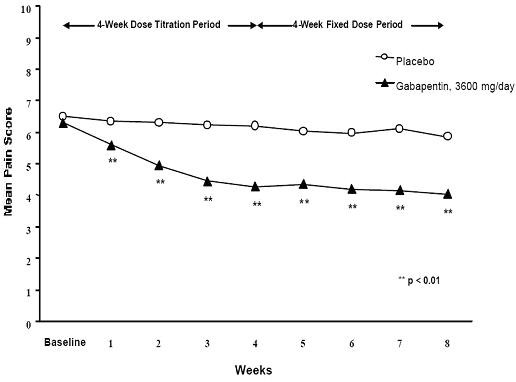 Figure 1. Weekly Mean Pain Scores (Observed Cases in ITT Population): Study 1
Figure 1. Weekly Mean Pain Scores (Observed Cases in ITT Population): Study 1 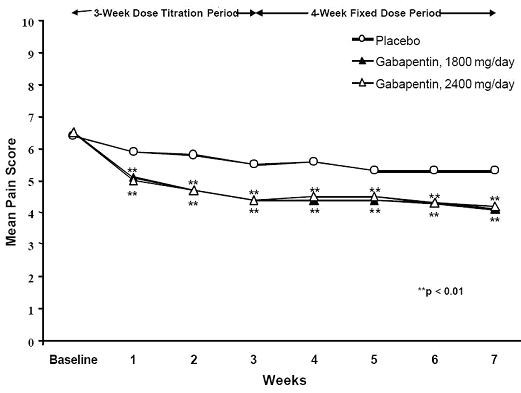 Figure 2. Weekly Mean Pain Scores (Observed Cases in ITT Population): Study 2
Figure 2. Weekly Mean Pain Scores (Observed Cases in ITT Population): Study 2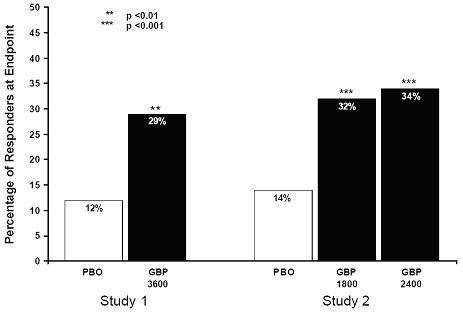 Figure 3. Proportion of Responders (patients with ≥50% reduction in pain score) at Endpoint: Controlled PHN Studies
Figure 3. Proportion of Responders (patients with ≥50% reduction in pain score) at Endpoint: Controlled PHN Studies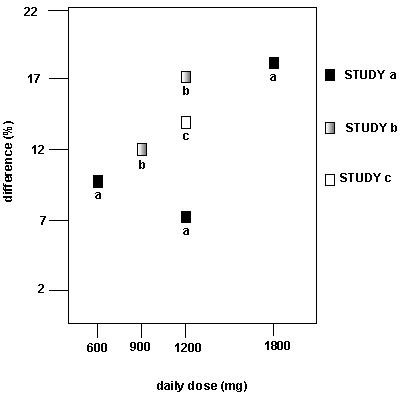 Figure 4. Responder Rate in Patients Receiving Gabapentin Expressed as a Difference from Placebo by Dose and Study: Adjunctive Therapy Studies in Patients ≥12 Years of Age with Partial Seizures
Figure 4. Responder Rate in Patients Receiving Gabapentin Expressed as a Difference from Placebo by Dose and Study: Adjunctive Therapy Studies in Patients ≥12 Years of Age with Partial SeizuresGABAPENTIN INDICATIONS AND USAGE
Postherpetic Neuralgia
Epilepsy
GABAPENTIN CONTRAINDICATIONS
Gabapentin tablets are contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients.
WARNINGS
Suicidal Behavior and Ideation
| Indication | Placebo Patients with Events Per 1000 Patients |
Drug Patients with Events Per 1000 Patients |
Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients |
Risk Difference: Additional Drug Patients with Events Per 1000 Patients |
|---|---|---|---|---|
| Epilepsy |
1 |
3.4 |
3.5 |
2.4 |
| Psychiatric |
5.7 |
8.5 |
1.5 |
2.9 |
| Other |
1 |
1.8 |
1.9 |
0.9 |
| Total |
2.4 |
4.3 |
1.8 |
1.9 |
Neuropsychiatric Adverse Events—Pediatric Patients 3 to 12 years of age
Withdrawal Precipitated Seizure, Status Epilepticus
Tumorigenic Potential
in vivo PRECAUTIONS: Carcinogenesis, Mutagenesis, Impairment of Fertility.
in situ
Sudden and Unexplained Death in Patients With Epilepsy
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
PRECAUTIONS
Information for Patients
Drug Interactions
PRECAUTIONS, Pregnancy
p
Laboratory Tests
Drug Interactions
In vitromax
maxmax
PRECAUTIONS
max
®
Drug/Laboratory Tests Interactions
®
Carcinogenesis, Mutagenesis, Impairment of Fertility
in vitro
in vitroin vivoin vitroin vitroin vivoin vivoin vivo
2
Pregnancy
Teratogenic Effects
Pregnancy Category C.
22
2222
2
in utero
Nursing Mothers
Gabapentin is secreted into human milk following oral administration. A nursed infant could be exposed to a maximum dose of approximately 1 mg/kg/day of gabapentin. Because the effect on the nursing infant is unknown, gabapentin should be used in women who are nursing only if the benefits clearly outweigh the risks.
Pediatric Use
CLINICAL PHARMACOLOGY, Clinical Studies
Geriatric Use
CLINICAL PHARMACOLOGY, ADVERSE REACTIONS, DOSAGE AND ADMINISTRATION
GABAPENTIN ADVERSE REACTIONS
Postherpetic Neuralgia
Incidence in Controlled Clinical Trials
| Body System/Preferred Term | Gabapentin N=336 % |
Placebo N=227 % |
|---|---|---|
| a Reported as blurred vision |
||
| Body as a Whole
|
||
| Asthenia |
5.7 |
4.8 |
| Infection |
5.1 |
3.5 |
| Headache |
3.3 |
3.1 |
| Accidental injury |
3.3 |
1.3 |
| Abdominal pain |
2.7 |
2.6 |
| Digestive System
|
||
| Diarrhea |
5.7 |
3.1 |
| Dry mouth |
4.8 |
1.3 |
| Constipation |
3.9 |
1.8 |
| Nausea |
3.9 |
3.1 |
| Vomiting |
3.3 |
1.8 |
| Flatulence |
2.1 |
1.8 |
| Metabolic and Nutritional Disorders
|
||
| Peripheral edema |
8.3 |
2.2 |
| Weight gain |
1.8 |
0 |
| Hyperglycemia |
1.2 |
0.4 |
| Nervous System
|
||
| Dizziness |
28 |
7.5 |
| Somnolence |
21.4 |
5.3 |
| Ataxia |
3.3 |
0 |
| Thinking abnormal |
2.7 |
0 |
| Abnormal gait |
1.5 |
0 |
| Incoordination |
1.5 |
0 |
| Amnesia |
1.2 |
0.9 |
| Hypesthesia |
1.2 |
0.9 |
| Respiratory System
|
||
| Pharyngitis |
1.2 |
0.4 |
| Skin and Appendages
|
||
| Rash |
1.2 |
0.9 |
| Special Senses
|
||
| Amblyopiaa
|
2.7 |
0.9 |
| Conjunctivitis |
1.2 |
0 |
| Diplopia |
1.2 |
0 |
| Otitis media |
1.2 |
0 |
Epilepsy
WARNINGS , Neuropsychiatric Adverse Events
Incidence in Controlled Clinical Trials
| Body System/Adverse Event | Gabapentina
N=543 % |
Placeboa
N=378 % |
|---|---|---|
| a Plus background antiepileptic drug therapy b Amblyopia was often described as blurred vision. |
||
| Body As A Whole
|
||
| Fatigue |
11 |
5 |
| Weight Increase |
2.9 |
1.6 |
| Back Pain |
1.8 |
0.5 |
| Peripheral Edema |
1.7 |
0.5 |
| Cardiovascular
|
||
| Vasodilatation |
1.1 |
0.3 |
| Digestive System
|
||
| Dyspepsia |
2.2 |
0.5 |
| Mouth or Throat Dry |
1.7 |
0.5 |
| Constipation |
1.5 |
0.8 |
| Dental Abnormalities |
1.5 |
0.3 |
| Increased Appetite |
1.1 |
0.8 |
| Hematologic and Lymphatic Systems
|
||
| Leukopenia |
1.1 |
0.5 |
| Musculoskeletal System
|
||
| Myalgia |
2 |
1.9 |
| Fracture |
1.1 |
0.8 |
| Nervous System
|
||
| Somnolence |
19.3 |
8.7 |
| Dizziness |
17.1 |
6.9 |
| Ataxia |
12.5 |
5.6 |
| Nystagmus |
8.3 |
4 |
| Tremor |
6.8 |
3.2 |
| Nervousness |
2.4 |
1.9 |
| Dysarthria |
2.4 |
0.5 |
| Amnesia |
2.2 |
0 |
| Depression |
1.8 |
1.1 |
| Thinking Abnormal |
1.7 |
1.3 |
| Twitching |
1.3 |
0.5 |
| Coordination Abnormal |
1.1 |
0.3 |
| Respiratory System
|
||
| Rhinitis |
4.1 |
3.7 |
| Pharyngitis |
2.8 |
1.6 |
| Coughing |
1.8 |
1.3 |
| Skin and Appendages
|
||
| Abrasion |
1.3 |
0 |
| Pruritus |
1.3 |
0.5 |
| Urogenital System
|
||
| Impotence |
1.5 |
1.1 |
| Special Senses
|
||
| Diplopia |
5.9 |
1.9 |
| Amblyopiab
|
4.2 |
1.1 |
| Laboratory Deviations
|
||
| WBC Decreased |
1.1 |
0.5 |
| Body System/Adverse Event | Gabapentina
N=119 % |
Placeboa
N=128 % |
|---|---|---|
| a Plus background antiepileptic drug therapy |
||
| Body As A Whole
|
||
| Viral Infection |
10.9 |
3.1 |
| Fever |
10.1 |
3.1 |
| Weight Increase |
3.4 |
0.8 |
| Fatigue |
3.4 |
1.6 |
| Digestive System
|
||
| Nausea and/or Vomiting |
8.4 |
7 |
| Nervous System
|
||
| Somnolence |
8.4 |
4.7 |
| Hostility |
7.6 |
2.3 |
| Emotional Lability |
4.2 |
1.6 |
| Dizziness |
2.5 |
1.6 |
| Hyperkinesia |
2.5 |
0.8 |
| Respiratory System
|
||
| Bronchitis |
3.4 |
0.8 |
| Respiratory Infection |
2.5 |
0.8 |
Other Adverse Events Observed During All Clinical Trials
Body As A Whole: Frequent: Infrequent: Rare:
Cardiovascular System: Frequent: Infrequent: Rare:
Digestive System: Frequent:Infrequent: Rare:
Endocrine System: Rare:
Hematologic and Lymphatic System: Frequent: Infrequent: Rare:
Musculoskeletal System: Frequent: Infrequent: Rare:
Nervous System: Frequent: Infrequent: Rare:
Respiratory System: Frequent:Infrequent: Rare:
Dermatological: Infrequent: Rare:
Urogenital System: Infrequent: Rare:
Special Senses: Frequent: Infrequent: Rare:
Body as a Whole:
Digestive System:
Hemic and Lymphatic System:
Nervous System:
Psychobiologic Function:
Respiratory System:
Body as a Whole: Infrequent:Rare:
Cardiovascular System: Infrequent:Rare:
Digestive System: Infrequent:Rare:
Endocrine System: Infrequent:
Hemic and Lymphatic System: Infrequent:Rare:
Metabolic and Nutritional: Infrequent:Rare:
Musculoskeletal: Infrequent:Rare:
Nervous System: Frequent:Infrequent:Rare:
Respiratory System: Infrequent:Rare:
Skin and Appendages: Infrequent: Rare:
Special Senses: Infrequent:Rare:
Urogenital System: Infrequent:Rare:, ,
Postmarketing and Other Experience
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
GABAPENTIN DOSAGE AND ADMINISTRATION
Postherpetic Neuralgia
Epilepsy
CLINICAL PHARMACOLOGY, Pediatrics
Dosage in Renal Impairment
Cr
CrCr
CrCr
Cr
| Renal Function Creatinine Clearance (mL/min) |
Total Daily Dose Range (mg/day) |
Dose Regimen (mg) |
||||
|---|---|---|---|---|---|---|
| a For patients with creatinine clearance <15 mL/min, reduce daily dose in proportion to creatinine clearance (e.g., patients with a creatinine clearance of 7.5 mL/min should receive one-half the daily dose that patients with a creatinine clearance of 15 mL/min receive). b Patients on hemodialysis should receive maintenance doses based on estimates of creatinine clearance as indicated in the upper portion of the table and a supplemental post-hemodialysis dose administered after each 4 hours of hemodialysis as indicated in the lower portion of the table. |
||||||
| ≥60 |
900-3600 |
300 TID |
400 TID |
600 TID |
800 TID |
1200 TID |
| >30-59 |
400-1400 |
200 BID |
300 BID |
400 BID |
500 BID |
700 BID |
| >15-29 |
200-700 |
200 QD |
300 QD |
400 QD |
500 QD |
700 QD |
| 15a
|
100-300 |
100 QD |
125 QD |
150 QD |
200 QD |
300 QD |
| Post-Hemodialysis Supplemental Dose (mg)b
|
||||||
| Hemodialysis |
125b
|
150b
|
200b
|
250b
|
350b
|
|
Dosage in Elderly
HOW SUPPLIED
Gabapentin Tablets USP, 600 mg are white, biconvex, elliptical, film-coated tablets, with deep break line on both sides and debossed with ‘D’ and ‘24’ on either side of the break line on one side and plain on other side.
Bottles of 10 NDC 33261-051-10
Bottles of 20 NDC 33261-051-20
Bottles of 21 NDC 33261-051-21
Bottles of 30 NDC 33261-051-30
Bottles of 42 NDC 33261-051-42
Bottles of 45 NDC 33261-051-45
Bottles of 60 NDC 33261-051-60
Bottles of 81 NDC 33261-051-81
Bottles of 90 NDC 33261-051-90
Bottles of 100 NDC 33261-051-00
Bottles of 120 NDC 33261-051-02
Bottles of 126 NDC 33261-051-97
Bottles of 180 NDC 33261-051-99
Gabapentin Tablets USP, 800 mg are white, biconvex, elliptical, film-coated tablets, with deep break line on both sides and debossed with ‘D’ and ‘25’ on either side of the break line on one side and plain on other side.
Bottles of 10 NDC 33261-116-10
Bottles of 20 NDC 33261-116-20
Bottles of 21 NDC 33261-116-21
Bottles of 30 NDC 33261-116-30
Bottles of 42 NDC 33261-116-42
Bottles of 45 NDC 33261-116-45
Bottles of 60 NDC 33261-116-60
Bottles of 81 NDC 33261-116-81
Bottles of 90 NDC 33261-116-90
Bottles of 100 NDC 33261-116-00
Bottles of 120 NDC 33261-116-02
Bottles of 126 NDC 33261-116-97
Bottles of 180 NDC 33261-116-99
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Maalox® is a registered trademark of Novartis Consumer Health, Inc.
Manufactured for:
Aurobindo Pharma USA, Inc.
2400 Route 130 North
Dayton, NJ 08810
Manufactured by:
Aurobindo Pharma Limited
Unit-VII (SEZ)
Mahaboob Nagar (Dt)
AP-509302, INDIA
Repackaged By:
Aidarex Pharmaceuticals, LLC.
Corona, CA 92880
Issued: August 2011
MEDICATION GUIDE
Gabapentin Tablets, USP
Rx only
What is the most important information I should know about gabapentin tablets?
Do not stop taking gabapentin tablets without first talking to your healthcare provider.
Gabapentin tablets can cause serious side effects including:
1. Like other antiepileptic drugs, gabapentin tablets may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Do not stop taking gabapentin tablets without first talking to a healthcare provider.
- Stopping gabapentin tablets suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
2. Changes in behavior and thinking
3. Gabapentin tablets may cause a serious or life-threatening allergic reaction
- skin rash
- hives
- fever
- swollen glands that do not go away
- swelling of your lip and tongue
- yellowing of your skin or of the whites of the eyes
- unusual bruising or bleeding
- severe fatigue or weakness
- unexpected muscle pain
- frequent infections
What are gabapentin tablets?
- Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults.
- Partial seizures when taken together with other medicines in adults and children 3 years of age and older.
Who should not take gabapentin tablets?
What should I tell my healthcare provider before taking gabapentin tablets?
Before taking gabapentin tablets, tell your healthcare provider if you:
- have or have had kidney problems or are on hemodialysis
- have or have had depression, mood problems, or suicidal thoughts or behavior
- are pregnant or plan to become pregnant. It is not known if gabapentin tablets can harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking gabapentin tablets. You and your healthcare provider will decide if you should take gabapentin tablets while you are pregnant.
- If you become pregnant while taking gabapentin tablets, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy. You can enroll in this registry by calling 1-888-233-2334.
- are breastfeeding or plan to breastfeed. Gabapentin can pass into breast milk. You and your healthcare provider should decide how you will feed your baby while you take gabapentin tablets.
Tell your healthcare provider about all the medicines you take,
How should I take gabapentin tablets?
- Take gabapentin tablets exactly as prescribed. Your healthcare provider will tell you how much gabapentin tablets to take.
- Do not change your dose of gabapentin tablets without talking to your healthcare provider. If you break a tablet in half the unused half of the tablet should be taken at your next scheduled dose. Half tablets not used within several days of breaking should be thrown away.
- Gabapentin tablets can be taken with or without food. If you take an antacid containing aluminum and magnesium, such as Maalox®, Mylanta®, Gelusil®, Gaviscon®, or Di-Gel®, you should wait at least 2 hours before taking your next dose of gabapentin tablets.
- If you take too much gabapentin tablets, call your healthcare provider or your local Poison Control Center right away.
What should I avoid while taking gabapentin tablets?
- Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking gabapentin tablets without first talking with your healthcare provider. Taking gabapentin tablets with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how gabapentin tablets affect you. Gabapentin tablets can slow your thinking and motor skills.
What are the possible side effects of gabapentin tablets?
- See “What is the most important information I should know about gabapentin tablets?”
-
The most common side effects of gabapentin tablets include:
- dizziness
- difficulty with speaking
- lack of coordination
- temporary loss of memory (amnesia)
- viral infection
- tremor
- feeling drowsy
- difficulty with coordination
- feeling tired
- double vision
- fever
- unusual eye movement
- jerky movements
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store gabapentin tablets?
- Store gabapentin tablets between 20° to 25°C (68° to 77°F).
Keep gabapentin tablets and all medicines out of the reach of children.
General information about the safe and effective use of gabapentin tablets
What are the ingredients in gabapentin tablets?
Active ingredient:
Inactive ingredients:
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 600 mg (100 Tablet Bottle)
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 800 mg (100 Tablet Bottle)

GabapentinGabapentin TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
GabapentinGabapentin TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||