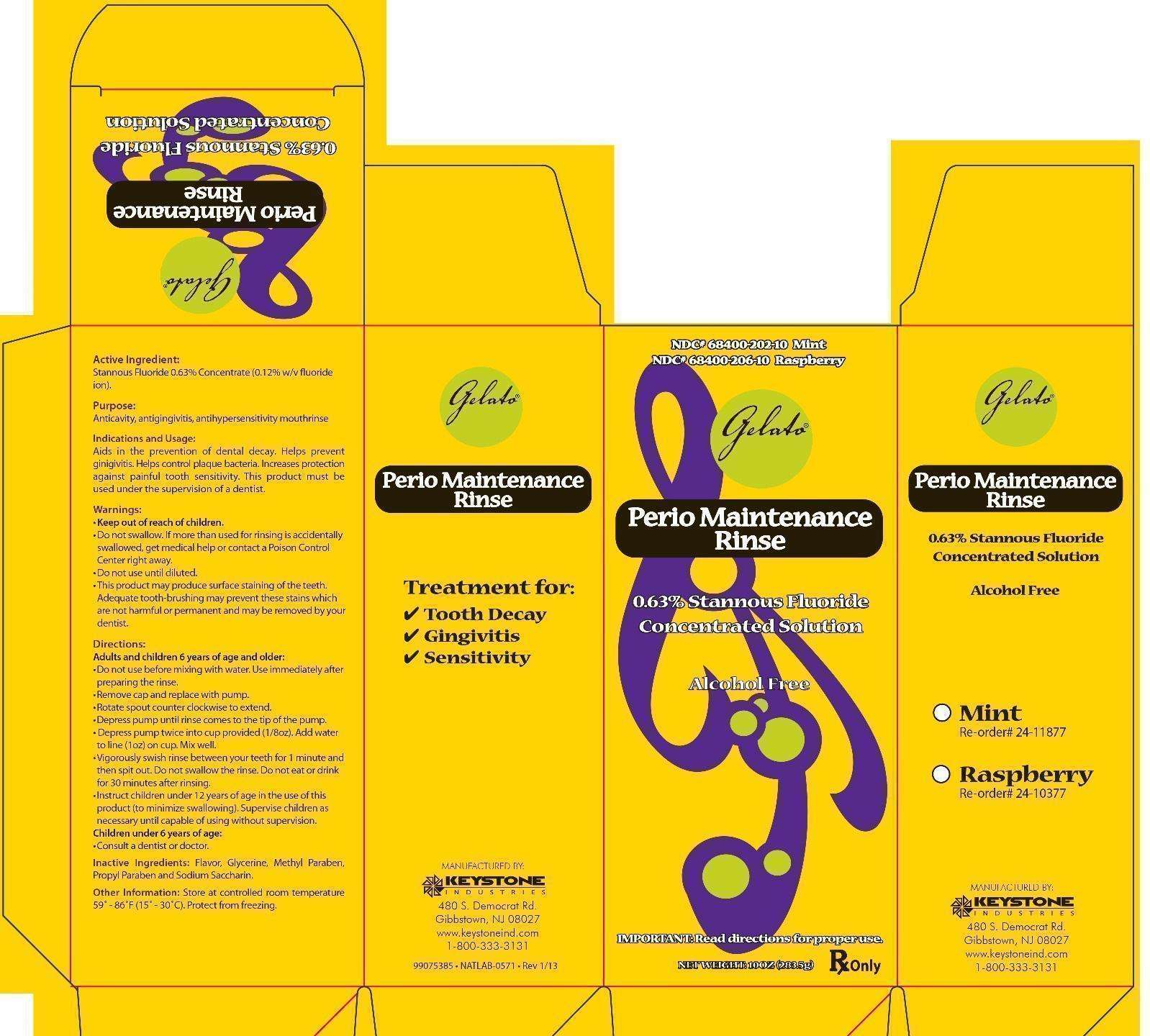
Gelato Perio Maintenance Rinse
Mycone Dental Supply Co., Inc DBA Keystone Industries and Deepak Products Inc.
Mycone Dental Supply Co., Inc DBA Keystone Industries and Deepak Products Inc
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active Ingredient:
Stannous Fluoride 0.63% Concentrate (0.12% w/v fluoride ion).
Uses
Keep out of reach of children.
Do not swallow. If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away. Do not use until diluted.
This product may produce surface staining of the teeth.
Adequate tooth-brushing may prevent these stains which are not harmful or permanent and may be removed by your dentist.
Directions:
Adults and children 6 years of age and older:
- Do not use before mixing with water. Use immediately after preparing the rinse.
- Remove cap and replace with pump.
- Rotate spout container clockwise to extend.
- Depress pump until rinse comes to the tip of the pump.
- Depress pump twice into cup provided (1/8oz). Add water to line (1oz) on cup. Mix well.
- Vigorously swish rinse between your teeth for 1 minute and then spit out. Do not swallow the rinse. Do not eat or drink for 30 minutes after rinsing.
- Instruct children under 12 years of age in the use of this product (to minimize swallowing). Supervise children as necessary until capable of using without supervision.
Children under 6 years of age:
- Consult a dentist or doctor.
Inactive Ingredients:
Flavor, Glycerin, Methyl paraben, Propyl paraben and Sodium Saccharin.
Other Information:
Store at controlled room temperature 59° - 86°F (15°-30°C). Protect from freezing.

Gelato Perio Maintenance RinseStannous Fluoride CONCENTRATE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||