GEMCITABINE
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use gemcitabine safely and effectively. See full prescribing information for gemcitabine for injection. Gemcitabine for Injection USP, Powder, Lyophilized, For Solution For Intravenous UseInitial U.S. Approval: 1996 INDICATIONS AND USAGEGemcitabine for injection is a nucleoside metabolic inhibitor indicated for: Ovarian cancer in combination with carboplatin (1.1) Breast cancer in combination with paclitaxel (1.2) Non-small cell lung cancer in combination with cisplatin (1.3) Pancreatic cancer as a single-agent (1.4) DOSAGE AND ADMINISTRATIONGemcitabine for injection is for intravenous use only. Ovarian cancer: 1000 mg/m2 over 30 minutes on Days 1 and 8 of each 21-day cycle (2.1) Breast cancer: 1250 mg/m2 over 30 minutes on Days 1 and 8 of each 21-day cycle (2.2) Non-small cell lung cancer: 4-week schedule, 1000 mg/m2 over 30 minutes on Days 1, 8, and 15 of each 28-day cycle: 3-week schedule; 1250 mg/m2 over 30 minutes on Days 1 and 8 of each 21-day cycle (2.3) Pancreatic cancer: 1000 mg/m2 over 30 minutes once weekly for up to 7 weeks (or until toxicity necessitates reducing or holding a dose), followed by a week of rest from treatment. Subsequent cycles should consist of infusions once weekly for 3 consecutive weeks out of every 4 weeks (2.4) Dose Reductions or discontinuation may be needed based on toxicities (2.1 to 2.4) DOSAGE FORMS AND STRENGTHS 200 mg vial for injection (3) 1 g vial for injection (3) CONTRAINDICATIONS4WARNINGS AND PRECAUTIONS Infusion time and dose frequency: Increased toxicity with infusion time >60 minutes or dosing more frequently than once weekly. (5.1) Hematology: Monitor for myelosuppression, which can be dose-limiting. (5.2, 5.7) Pulmonary toxicity: Discontinue gemcitabine immediately for severe pulmonary toxicity. (5.3) Renal: Monitor renal function prior to initiation of therapy and periodically thereafter. Use with caution in patients with renal impairment. Cases of hemolytic uremic syndrome (HUS) and/or renal failure, some fatal, have occurred. Discontinue gemcitabine for HUS or severe renal toxicity (5.4) Hepatic: Monitor hepatic function prior to initiation of therapy and periodically thereafter. Use with caution in patients with hepatic impairment. Serious hepatotoxicity, including liver failure and death, have occurred. Discontinue gemcitabine for severe hepatic toxicity. (5.5) Pregnancy: Can cause fetal harm. Advise women of potential risk to the fetus. (5.6, 8.1) Radiation toxicity. May cause severe and life-threatening toxicity (5.8) Side Effects6.1 To report SUSPECTED ADVERSE REACTIONS, contact CARACO Pharmaceutical Laboratories Ltd. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 GEMCITABINE INDICATIONS AND USAGE
- 2 GEMCITABINE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 GEMCITABINE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 GEMCITABINE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 GEMCITABINE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
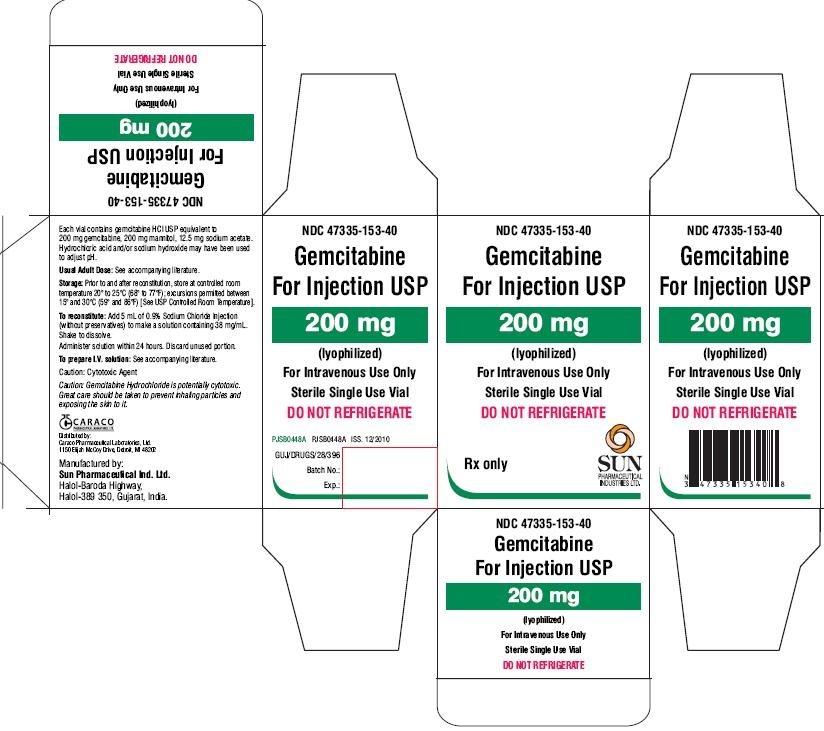
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL 200MG
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON 200MG
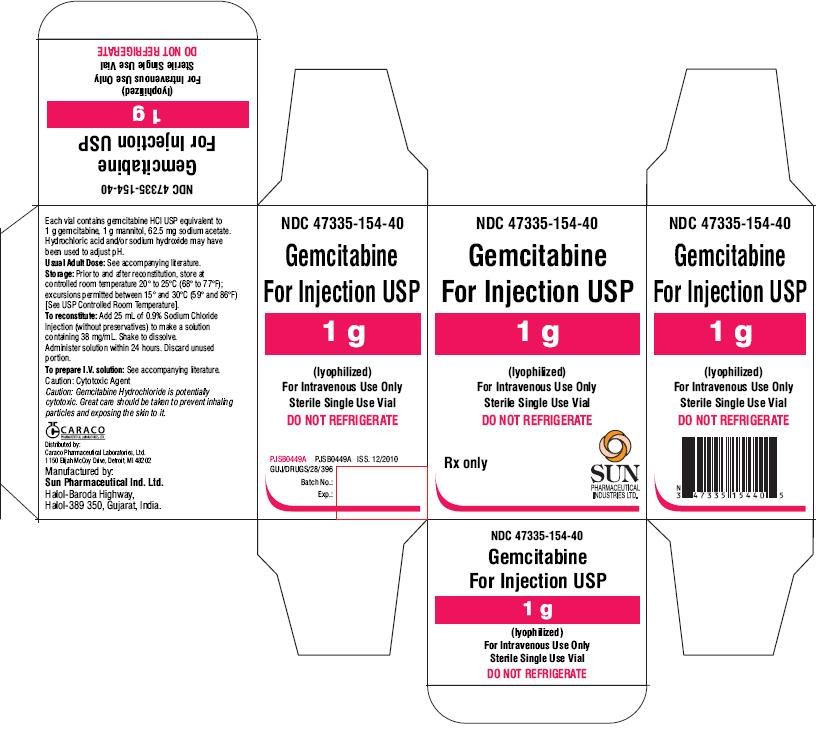
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL 1G
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON 1G
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Ovarian Cancer
1.2 Breast Cancer
1.3 Non-Small Cell Lung Cancer
1.4 Pancreatic Cancer
2 DOSAGE AND ADMINISTRATION
2.1 Ovarian Cancer
2 66
Dose Modifications
| Absolute granulocyte count (x 106/L) |
Platelet count (x 106/L) |
% of full dose | |
|---|---|---|---|
| ≥1500 |
And |
≥100,000 |
100 |
| 1000 to 1499 |
and/or |
75,000 to 99,999 |
50 |
| <1000 |
and/or |
<75,000 |
Hold |
2
- Absolute granulocyte count <500 x 106/L for more than 5 days
- Absolute granulocyte count <100 x 106/L for more than 3 days
- Febrile neutropenia
- Platelets <25,000 x 106/L
- Cycle delay of more than one week due to toxicity
2.2 Breast Cancer
2 2 66
Dose Modifications
| Absolute granulocyte count (x 106/L) |
Platelet count (x 106/L) |
% of full dose | |
|---|---|---|---|
| ≥1200 |
And |
>75,000 |
100 |
| 1000 to 1199 |
Or |
50,000 to 75,000 |
75 |
| 700 to 999 |
And |
≥50,000 |
50 |
| <700 |
Or |
<50,000 |
Hold |
2.3 Non-Small Cell Lung Cancer
[see Clinical Studies (14.3)]2 2 2 2
Dose Modifications
2.4 Pancreatic Cancer
2
Dose Modifications
[see Warnings and Precautions (5.2)][see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)]
| Absolute granulocyte count (x 106/L) |
Platelet count (x 106/L) |
% of full dose | |
|---|---|---|---|
| ≥1000 |
And |
≥100,000 |
100 |
| 500 to 999 |
Or |
50,000 to 99,999 |
75 |
| <500 |
Or |
<50,000 |
Hold |
6666
2.5 Preparation and Administration Precautions
[see References (15)]
2.6 Preparation for Intravenous Infusion Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Infusion Time
[see Clinical Studies (14.5)]
5.2 Hematology
[see Adverse Reactions (6.1)][see Dosage and Administration (2.1, 2.2, 2.3, and 2.4)].
5.3 Pulmonary
[see Adverse Reactions (6.1 and 6.2)]
5.4 Renal
[see Adverse Reactions (6.1 and 6.2)]
[see Use in Specific Populations (8.6)].
5.5 Hepatic
[see Adverse Reactions (6.1 and 6.2)]
[see Use in Specific Populations (8.7)].
5.6 Pregnancy
[see Use In Specific Populations (8.1)].
5.7 Laboratory Tests
[see Dosage and Administration (2.1, 2.2, 2.3, and 2.4)] [see Dosage and Administration (2.4)]
5.8 Radiation Therapy
Non-concurrent (given >7 days apart)
Concurrent (given together or ≤7 days apart) 2 3
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Single-Agent Use[see Dosage and Administration (2.1, 2.2, 2.3, and 2.4)]
2
All Patients |
Pancreatic Cancer Patients |
Discontinuations (%) |
|||||
|---|---|---|---|---|---|---|---|
| All Grades | Grade 3 | Grade 4 | All Grades | Grade 3 | Grade 4 | All Patients | |
Laboratory |
|||||||
| Hematologic
|
|||||||
| Anemia
|
68 |
7 |
1 |
73 |
8 |
2 |
<1 |
| Leukopenia
|
62 |
9 |
<1 |
64 |
8 |
1 |
<1 |
| Neutropenia
|
63 |
19 |
6 |
61 |
17 |
7 |
- |
| Thrombocytopenia
|
24 |
4 |
1 |
36 |
7 |
<1 |
<1 |
| Hepatic |
<1 |
||||||
| ALT |
68 |
8 |
2 |
72 |
10 |
1 |
|
| AST |
67 |
6 |
2 |
78 |
12 |
5 |
|
| Alkaline Phosphatase |
55 |
7 |
2 |
77 |
16 |
4 |
|
| Bilirubin |
13 |
2 |
<1 |
26 |
6 |
2 |
|
| Renal |
<1 |
||||||
| Proteinuria |
45 |
<1 |
0 |
32 |
<1 |
0 |
|
| Hematuria |
35 |
<1 |
0 |
23 |
0 |
0 |
|
| BUN |
16 |
0 |
0 |
15 |
0 |
0 |
|
| Creatinine |
8 |
<1 |
0 |
6 |
0 |
0 |
|
Non-laboratory |
|||||||
| Nausea and Vomiting |
69 |
13 |
1 |
71 |
10 |
2 |
<1 |
| Fever |
41 |
2 |
0 |
38 |
2 |
0 |
<1 |
| Rash |
30 |
<1 |
0 |
28 |
<1 |
0 |
<1 |
| Dyspnea |
23 |
3 |
<1 |
10 |
0 |
<1 |
<1 |
| Diarrhea |
19 |
1 |
0 |
30 |
3 |
0 |
0 |
| Hemorrhage |
17 |
<1 |
<1 |
4 |
2 |
<1 |
<1 |
| Infection |
16 |
1 |
<1 |
10 |
2 |
<1 |
<1 |
| Alopecia |
15 |
<1 |
0 |
16 |
0 |
0 |
0 |
| Stomatitis |
11 |
<1 |
0 |
10 |
<1 |
0 |
<1 |
| Somnolence |
11 |
<1 |
<1 |
11 |
2 |
<1 |
<1 |
| Paresthesias |
10 |
<1 |
0 |
10 |
<1 |
0 |
0 |
Hematologic [see Dosage and Administration (2.1, 2.2, 2.3, and 2.4)]
Gastrointestinal
Hepatic [see Adverse Reactions (6.2)]
Renal [see Adverse Reactions (6.2)]
Fever
Rash
Pulmonary [see Adverse Reactions (6.2)]
Edema
Flu-like Symptoms
Infection
Alopecia
Neurotoxicity
Extravasation
Allergic [see Contraindications (4)]
Cardiovascular [see Adverse Reactions (6.2)]
Combination Use in Non-Small Cell Lung Cancer:
CTC Grades (% incidence) |
||||||
|---|---|---|---|---|---|---|
Gemcitabine plus Cisplatin |
Cisplatin |
|||||
| All Grades | Grade 3 | Grade 4 | All Grades | Grade 3 | Grade 4 | |
Laboratory |
||||||
| Hematologic |
||||||
| Anemia |
89 |
22 |
3 |
67 |
6 |
1 |
RBC Transfusion |
39 |
13 |
||||
| Leukopenia |
82 |
35 |
11 |
25 |
2 |
1 |
| Neutropenia |
79 |
22 |
35 |
20 |
3 |
1 |
| Thrombocytopenia |
85 |
25 |
25 |
13 |
3 |
1 |
Platelet Transfusions |
21 |
<1 |
||||
| Lymphocytes |
75 |
25 |
18 |
51 |
12 |
5 |
| Hepatic |
||||||
| Transaminase |
22 |
2 |
1 |
10 |
1 |
0 |
| Alkaline Phosphatase |
19 |
1 |
0 |
13 |
0 |
0 |
| Renal |
||||||
| Proteinuria |
23 |
0 |
0 |
18 |
0 |
0 |
| Hematuria |
15 |
0 |
0 |
13 |
0 |
0 |
| Creatinine |
38 |
4 |
<1 |
31 |
2 |
<1 |
| Other Laboratory |
||||||
| Hyperglycemia |
30 |
4 |
0 |
23 |
3 |
0 |
| Hypomagnesemia |
30 |
4 |
3 |
17 |
2 |
0 |
| Hypocalcemia |
18 |
2 |
0 |
7 |
0 |
<1 |
Non-laboratory |
||||||
| Nausea |
93 |
25 |
2 |
87 |
20 |
<1 |
| Vomiting |
78 |
11 |
12 |
71 |
10 |
9 |
| Alopecia |
53 |
1 |
0 |
33 |
0 |
0 |
| Neuro Motor |
35 |
12 |
0 |
15 |
3 |
0 |
| Neuro Hearing |
25 |
6 |
0 |
21 |
6 |
0 |
| Diarrhea |
24 |
2 |
2 |
13 |
0 |
0 |
| Neuro Sensory |
23 |
1 |
0 |
18 |
1 |
0 |
| Infection |
18 |
3 |
2 |
12 |
1 |
0 |
| Fever |
16 |
0 |
0 |
5 |
0 |
0 |
| Neuro Cortical |
16 |
3 |
1 |
9 |
1 |
0 |
| Neuro Mood |
16 |
1 |
0 |
10 |
1 |
0 |
| Local |
15 |
0 |
0 |
6 |
0 |
0 |
| Neuro Headache |
14 |
0 |
0 |
7 |
0 |
0 |
| Stomatitis |
14 |
1 |
0 |
5 |
0 |
0 |
| Hemorrhage |
14 |
1 |
0 |
4 |
0 |
0 |
| Dyspnea |
12 |
4 |
3 |
11 |
3 |
2 |
| Hypotension |
12 |
1 |
0 |
7 |
1 |
0 |
| Rash |
11 |
0 |
0 |
3 |
0 |
0 |
WHO Grades (% incidence) |
||||||
|---|---|---|---|---|---|---|
Gemcitabine plus Cisplatin |
Etoposide plus Cisplatin |
|||||
| All Grades | Grade 3 | Grade 4 | All Grades | Grade 3 | Grade 4 | |
Laboratory |
||||||
| Hematologic |
||||||
| Anemia |
88 |
22 |
0 |
77 |
13 |
2 |
RBC Transfusions |
29 |
21 |
||||
| Leukopenia |
86 |
26 |
3 |
87 |
36 |
7 |
| Neutropenia |
88 |
36 |
28 |
87 |
20 |
56 |
| Thrombocytopenia |
81 |
39 |
16 |
45 |
8 |
5 |
Platelet Transfusions |
3 |
8 |
||||
| Hepatic |
||||||
| ALT |
6 |
0 |
0 |
12 |
0 |
0 |
| AST |
3 |
0 |
0 |
11 |
0 |
0 |
| Alkaline Phosphatase |
16 |
0 |
0 |
11 |
0 |
0 |
| Bilirubin |
0 |
0 |
0 |
0 |
0 |
0 |
| Renal |
||||||
| Proteinuria |
12 |
0 |
0 |
5 |
0 |
0 |
| Hematuria |
22 |
0 |
0 |
10 |
0 |
0 |
| BUN |
6 |
0 |
0 |
4 |
0 |
0 |
| Creatinine |
2 |
0 |
0 |
2 |
0 |
0 |
Non-laboratory  |
||||||
| Nausea and Vomiting |
96 |
35 |
4 |
86 |
19 |
7 |
| Fever |
6 |
0 |
0 |
3 |
0 |
0 |
| Rash |
10 |
0 |
0 |
3 |
0 |
0 |
| Dyspnea |
1 |
0 |
1 |
3 |
0 |
0 |
| Diarrhea |
14 |
1 |
1 |
13 |
0 |
2 |
| Hemorrhage |
9 |
0 |
3 |
3 |
0 |
3 |
| Infection |
28 |
3 |
1 |
21 |
8 |
0 |
| Alopecia |
77 |
13 |
0 |
92 |
51 |
0 |
| Stomatitis |
20 |
4 |
0 |
18 |
2 |
0 |
| Somnolence |
3 |
0 |
0 |
3 |
2 |
0 |
| Paresthesias |
38 |
0 |
0 |
16 |
2 |
0 |
| CTC Grades (% incidence) | ||||||
|---|---|---|---|---|---|---|
| Gemcitabine plus Paclitaxel (N=262) | Paclitaxel (N=259) |
|||||
| All Grades | Grade 3 | Grade 4 | All Grades | Grade 3 | Grade 4 | |
Laboratory |
||||||
| Hematologic |
||||||
| Anemia |
69 |
6 |
1 |
51 |
3 |
<1 |
| Neutropenia |
69 |
31 |
17 |
31 |
4 |
7 |
| Thrombocytopenia |
26 |
5 |
<1 |
7 |
<1 |
<1 |
| Leukopenia |
21 |
10 |
1 |
12 |
2 |
0 |
| Hepatobiliary |
||||||
| ALT |
18 |
5 |
<1 |
6 |
<1 |
0 |
| AST |
16 |
2 |
0 |
5 |
<1 |
0 |
Non-laboratory |
||||||
| Alopecia |
90 |
14 |
4 |
92 |
19 |
3 |
| Neuropathy-sensory |
64 |
5 |
<1 |
58 |
3 |
0 |
| Nausea |
50 |
1 |
0 |
31 |
2 |
0 |
| Fatigue |
40 |
6 |
<1 |
28 |
1 |
<1 |
| Myalgia |
33 |
4 |
0 |
33 |
3 |
<1 |
| Vomiting |
29 |
2 |
0 |
15 |
2 |
0 |
| Arthralgia |
24 |
3 |
0 |
22 |
2 |
<1 |
| Diarrhea |
20 |
3 |
0 |
13 |
2 |
0 |
| Anorexia |
17 |
0 |
0 |
12 |
<1 |
0 |
| Neuropathy-motor |
15 |
2 |
<1 |
10 |
<1 |
0 |
| Stomatitis/pharyngitis |
13 |
1 |
<1 |
8 |
<1 |
0 |
| Fever |
13 |
<1 |
0 |
3 |
0 |
0 |
| Rash/desquamation |
11 |
<1 |
<1 |
5 |
0 |
0 |
Combination Use in Ovarian Cancer:
| CTC Grades (% incidence) | ||||||
|---|---|---|---|---|---|---|
| Gemcitabine plus Carboplatin (N=175) | Carboplatin (N=174) |
|||||
| All Grades | Grade 3 | Grade 4 | All Grades | Grade 3 | Grade 4 | |
Laboratory |
||||||
| Hematologic
|
||||||
| Neutropenia |
90 |
42 |
29 |
58 |
11 |
1 |
| Anemia |
86 |
22 |
6 |
75 |
9 |
2 |
| Leukopenia |
86 |
48 |
5 |
70 |
6 |
<1 |
| Thrombocytopenia |
78 |
30 |
5 |
57 |
10 |
1 |
RBC Transfusions |
38 |
15 |
||||
| Platelet Transfusions |
9 |
3 |
||||
|
Non-laboratory
|
||||||
| Nausea
|
69 |
6 |
0 |
61 |
3 |
0 |
| Alopecia |
49 |
0 |
0 |
17 |
0 |
0 |
| Vomiting |
46 |
6 |
0 |
36 |
2 |
<1 |
| Constipation |
42 |
6 |
1 |
37 |
3 |
0 |
| Fatigue |
40 |
3 |
<1 |
32 |
5 |
0 |
| Neuropathy-sensory |
29 |
1 |
0 |
27 |
2 |
0 |
| Diarrhea |
25 |
3 |
0 |
14 |
<1 |
0 |
| Stomatitis/pharyngitis |
22 |
<1 |
0 |
13 |
0 |
0 |
| Anorexia |
16 |
1 |
0 |
13 |
0 |
0 |
6.2 Postmarketing Experience
Cardiovascular
Vascular Disorders Skin
Hepatic
Pulmonary
Renal
Injury, Poisoning, and Procedural Complications [see Warnings and Precautions (5.8)]
7 DRUG INTERACTIONS
[see Clinical Pharmacology (12.2 and 12.3)]
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
2 2 [see Warnings and Precautions (5.6)].
8.3 Nursing Mothers
8.4 Pediatric Use
22
8.5 Geriatric Use
[see Clinical Pharmacology (12.3)][see Dosage and Administration (2.1, 2.2, 2.3, and 2.4)] [see Clinical Studies (14.1)]
8.6 Renal
[see Adverse Reactions (6.1 and 6.2)][see Warnings and Precautions (5.4)].
8.7 Hepatic
[see Adverse Reactions (6.1 and 6.2)][see Warnings and Precautions (5.5)].
8.8 Gender
[see Clinical Pharmacology (12.3)]. [see Dosage and Administration (2)]
10 OVERDOSAGE
2
11 DESCRIPTION
 911234
91123412 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
in vitroIn vivo
12.3 Pharmacokinetics
Absorption and Distribution
22 2
Metabolism
2
Excretion
| Age | Clearance Men (L/hr/m2) | Clearance Women (L/hr/m2) | Half-Life Men (min) |
Half-Life Women (min) |
|---|---|---|---|---|
| 29 |
92.2 |
69.4 |
42 |
49 |
| 45 |
75.7 |
57 |
48 |
57 |
| 65 |
55.1 |
41.5 |
61 |
73 |
| 79 |
40.7 |
30.7 |
79 |
94 |
Drug Interactions
2 2 2 22 [see Drug Interactions (7)]
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
in vitro in vivo in vivo in vitro in vitro2 2 2
14 CLINICAL STUDIES
14.1 Ovarian Cancer
2
| Gemcitabine/Carboplatin | Carboplatin | |
|---|---|---|
| Number of randomized patients |
178 |
178 |
| Median age, years |
59 |
58 |
| Range |
36 to 78 |
21 to 81 |
Baseline ECOG performance status 0 to 1 |
94% |
95% |
| Disease Status |
||
| Evaluable |
7.9% |
2.8% |
| Bidimensionally measurable |
91.6% |
95.5% |
Platinum-free interval |
||
| 6 to 12 months |
39.9% |
39.9% |
| >12 months |
59% |
59.6% |
| First-line therapy |
||
| Platinum-taxane combination |
70.2% |
71.3% |
| Platinum-non-taxane combination |
28.7% |
27.5% |
| Platinum monotherapy |
1.1% |
1.1% |
| Gemcitabine/Carboplatin (N=178) | Carboplatin (N=178) | ||
|---|---|---|---|
| PFS Median (95%, C.I.) months Hazard Ratio (95%, C.I.) |
8.6 (8, 9.7) |
5.8 (5.2, 7.1) |
p=0.0038 |
| 0.72 (0.57, 0.9) |
|||
| Overall Survival Median (95%, C.I.) months Hazard Ratio (95%, C.I.) Adjusted  (95%, C.I.) |
18 (16.2, 20.3) |
17.3 (15.2, 19.3) |
p=0.8977 |
| 0.98 (0.78, 1.24) |
|||
| 0.86 (0.67, 1.1) |
|||
| Investigator Reviewed Overall Response Rate CR PR+PRNM  |
47.2% 14.6% 32.6% |
30.9% 6.2% 24.7% |
p=0.0016  |
| Independently Reviewed Overall Response Rate   CR PR+PRNM |
46.3% 9.1% 37.2% |
35.6% 4% 31.7% |
p=0.11  |
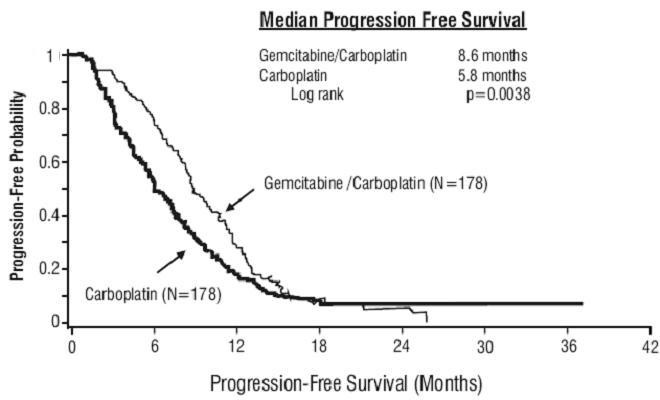 Figure 1: Kaplan-Meier Curve of Progression Free Survival in Gemcitabine Plus Carboplatin Versus Carboplatin in Ovarian Cancer (N=356)
Figure 1: Kaplan-Meier Curve of Progression Free Survival in Gemcitabine Plus Carboplatin Versus Carboplatin in Ovarian Cancer (N=356)14.2 Breast Cancer
222
| Gemcitabine/Paclitaxel | Paclitaxel | ||||
|---|---|---|---|---|---|
| Number of patients |
267 |
262 |
|||
| Median age, years Range |
53 26 to 83 |
52 26 to 75 |
|||
| Metastatic disease |
97% |
96.9% |
|||
Baseline KPS |
70.4% |
74.4% |
|||
| Number of tumor sites 1 to 2 ≥3 |
56.6% 43.4% |
58.8% 41.2% |
|||
| Visceral disease |
73.4% |
72.9% |
|||
| Prior anthracycline |
96.6% |
95.8% |
|||
Overall Survival |
|||||
| Median (95%, CI) |
18.6 (16.5, 20.7) |
15.8 (14.1, 17.3) |
|||
| Hazard Ratio (95%, CI) | 0.86 (0.71, 1.04) |
||||
Time to Documented Disease Progression Median (95%, C.I.), months Hazard Ratio (95%, C.I.) |
5.2 (4.2, 5.6) |
2.9 (2.6, 3.7) |
p<0.0001 |
||
| 0.65 (0.524, 0.805) |
p<0.0001 |
||||
| Overall Response Ratec (95%, C.I.) |
40.8% (34.9, 46.7) |
22.1% (17.1, 27.2) |
p<0.0001 |
||
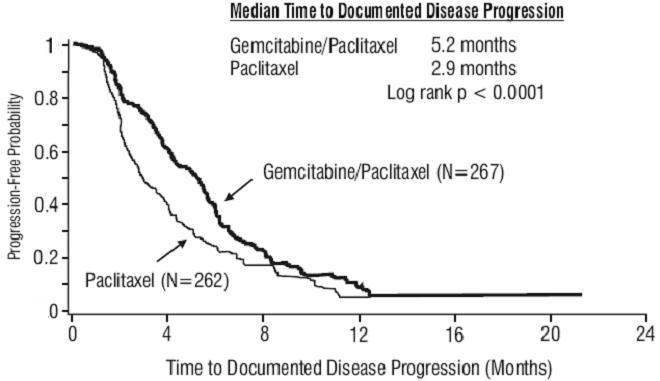 Figure 2: Kaplan-Meier Curve of Time to Documented Disease Progression in Gemcitabine Plus Paclitaxel Versus Paclitaxel Breast Cancer Study (N=529)
Figure 2: Kaplan-Meier Curve of Time to Documented Disease Progression in Gemcitabine Plus Paclitaxel Versus Paclitaxel Breast Cancer Study (N=529)14.3 Non-Small Cell Lung Cancer (NSCLC)
Gemcitabine plus cisplatin versus cisplatin:
2 2 2
Gemcitabine plus cisplatin versus etoposide plus cisplatin:
2 2 2 2
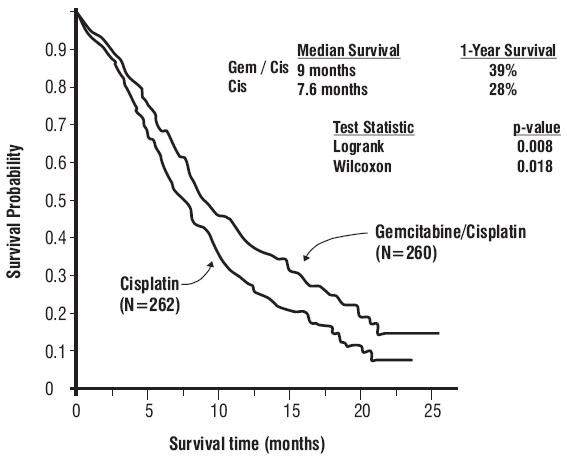
Figure 3: Kaplan-Meier Survival Curve in Gemcitabine Plus Cisplatin Versus Cisplatin NSCLC Study (N=522)
| Trial | 28-day Schedule |
21-day Schedule |
||||
|---|---|---|---|---|---|---|
| Treatment Arm | Gemcitabine/Cisplatin | Cisplatin | Gemcitabine/Cisplatin | Cisplatin/Etoposide | ||
| Number of patients Male Female |
260 182 78 |
262 186 76 |
|
69 64 5 |
66 61 5 |
|
| Median age, years Range |
62 36 to 88 |
63 35 to 79 |
|
58 33 to 76 |
60 35 to 75 |
|
| Stage IIIA Stage IIIB Stage IV |
7% 26% 67% |
7% 23% 70% |
|
N/A 48% 52% |
N/A 52% 49% |
|
Baseline KPS Baseline KPS  |
41% 57% |
44% 55% |
|
45% 55% |
52% 49% |
|
| Survival Median, months (95%, C.I.) months |
9 8.2, 11 |
7.6 6.6, 8.8 |
p=0.008 |
8.7 7.8, 10.1 |
7 6, 9.7 |
p=0.18 |
| Time to Disease Progression Median, months (95%, C.I.) months |
5.2 4.2, 5.7 |
3.7 3, 4.3 |
p=0.009 |
5 4.2, 6.4 |
4.1 2.4, 4.5 |
p=0.015 |
| Tumor Response |
26% |
10% |
p<0.0001  |
33% |
14% |
p=0.01  |
14.4 Pancreatic Cancer
2
2
| Gemcitabine | 5-FU | ||
|---|---|---|---|
| Number of patients Male Female |
63 34 29 |
63 34 29 |
|
| Median age Range |
62 years 37 to 79 |
61 years 36 to 77 |
|
| Stage IV disease |
71.4% |
76.2% |
|
Baseline KPS |
69.8% |
68.3% |
|
| Clinical benefit response |
22.2% (N  |
4.8% (N  |
p=0.004 |
| Survival Median 6-month probability  9-month probability  1-year probability  Range 95% C.I. of the median |
5.7 months (N=30) 46% (N=14) 24% (N=9) 18% 0.2 to 18.6 months 4.7 to 6.9 months |
4.2 months (N=19) 29% (N=4) 5% (N=2) 2% 0.4 to 15.1+  3.1 to 5.1 months |
p=0.0009 |
| Time to Disease Progression Median Range 95% C.I. of the median |
2.1 months 0.1+  1.9 to 3.4 months |
0.9 months 0.1 to 12+  0.9 to 1.1 months |
p=0.0013 |
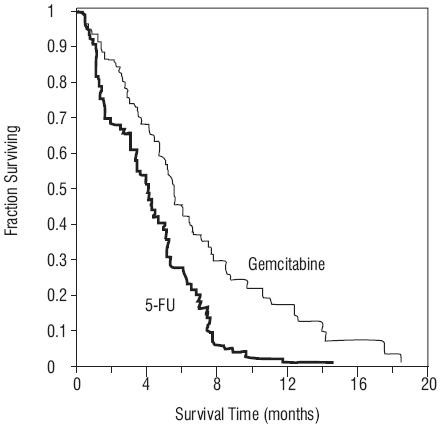 Figure 4: Kaplan-Meier Survival Curve
Figure 4: Kaplan-Meier Survival Curve14.5 Other Clinical Studies
22 2 2 [see Clinical Pharmacology (12.3)] [see Warnings and Precautions (5.1)]
15 REFERENCES
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165. 2. OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter
- Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- American Society of Health-System Pharmacists. ASHP Guidelines on Handling Hazardous Drugs: Am J Health-Syst Pharm. 2006;63:1172-1193.
- Polovich, M., White, J. M., & Kelleher, L. O. (eds.) 2005. Chemotherapy and biotherapy guidelines and recommendations for practice (2nd. ed.) Pittsburgh, PA: Oncology Nursing Society.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
[see Dosage and Administration (2.5 and 2.6)]
17 PATIENT COUNSELING INFORMATION
17.1 Low Blood Cell Counts
[see Warnings and Precautions (5.2)]
17.2 Pregnancy
[see Warnings and Precautions (5.6) and Use in Specific Populations (8.1)].
17.3 Nursing Mothers
[see Use in Specific Populations (8.3)].
Distributed by:
Caraco Pharmaceutical Laboratories, Ltd.
1150 Elijah McCoy Drive, Detroit, MI 48202
Manufactured by:
Sun Pharmaceutical Ind. Ltd.
Halol-Baroda Highway,
Halol-389 350, Gujarat
India.
ISS. 05/2011
PJPI0100B
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL 200MG
NDC 47335-153-40
Gemcitabine For Injection USP
200 mg
(lyophilized)
For Intravenous Use Only
Sterile Single Use Vial
Rx only

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON 200MG
NDC 47335-153-40
Gemcitabine For Injection USP
200 mg
(lyophilized)
For Intravenous Use Only
Sterile Single Use Vial
DO NOT REFRIGERATE
Rx only
SUN PHARMACEUTICAL INDUSTRIES LTD.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL 1G
NDC 47335-154-40
Gemcitabine For Injection USP
1 g
(lyophilized)
For Intravenous Use Only
Sterile Single Use Vial
Rx only

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON 1G
NDC 47335-154-40
Gemcitabine For Injection USP
1 g
(lyophilized)
For Intravenous Use Only
Sterile Single Use Vial
DO NOT REFRIGERATE
Rx only
SUN PHARMACEUTICAL INDUSTRIES LTD.
GEMCITABINEGEMCITABINE HYDROCHLORIDE INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
GEMCITABINEGEMCITABINE HYDROCHLORIDE INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!