Gemfibrozil
FULL PRESCRIBING INFORMATION: CONTENTS*
- GEMFIBROZIL DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- GEMFIBROZIL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- GEMFIBROZIL ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- OVERDOSAGE
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
GEMFIBROZIL DESCRIPTION
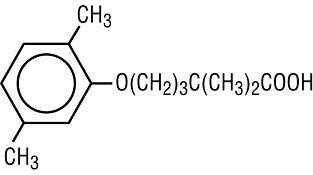
CLINICAL PHARMACOLOGY
Table I: Reduction in CHD Rates (events per 1000 patients) by Baseline Lipids *in the Helsinki Heart Study, Years 0 to 5
*
Table I).
Table II.
Table II: Cardiac Events and All-Cause Mortality (events per 1000 patients) Occurring During the 3.5 Year Open-Label Follow-up to the Helsinki Heart Study *
*
Table III). This analysis of the originally randomized "intent-to-treat" population neglects the possible complicating effects of treatment switching during the open-label phase. Adjustment of hazard ratios, taking into account open-label treatment status from years 6.5 to 8.5, could change the reported hazard ratios for mortality toward unity.
Table III: Cardiac Events, Cardiac Deaths, Non-Cardiac Deaths, and All-Cause Mortality in the Helsinki Heart Study, Years 0 to 8.5 *
*
DOSAGE AND ADMINISTRATION).
PRECAUTIONS).
INDICATIONS & USAGE
onlyin Type IIb patients without history of or symptoms of existing coronary heart disease who have had an inadequate response to weight loss, dietary therapy, exercise, and other pharmacologic agents (such as bile acid sequestrants and nicotinic acid, known to reduce LDL- and raise HDL-cholesterol)andwho have the following triad of lipid abnormalities: low HDL-cholesterol levels in addition to elevated LDL-cholesterol and elevated triglycerides (seeWARNINGS, PRECAUTIONS,andCLINICAL PHARMACOLOGY). The National Cholesterol Education Program has defined a serum HDL-cholesterol value that is consistently below 35 mg/dL as constituting an independent risk factor for coronary heart disease. Patients with significantly elevated triglycerides should be closely observed when treated with gemfibrozil. In some patients with high triglyceride levels, treatment with gemfibrozil is associated with a significant increase in LDL-cholesterol. BECAUSE OF POTENTIAL TOXICITY SUCH AS MALIGNANCY, GALLBLADDER DISEASE, ABDOMINAL PAIN LEADING TO APPENDECTOMY AND OTHER ABDOMINAL SURGERIES, AN INCREASED INCIDENCE IN NON-CORONARY MORTALITY, AND THE 44% RELATIVE INCREASE DURING THE TRIAL PERIOD IN AGE-ADJUSTED ALL-CAUSE MORTALITY SEEN WITH THE CHEMICALLY AND PHARMACOLOGICALLY RELATED DRUG, CLOFIBRATE, THE POTENTIAL BENEFIT OF GEMFIBROZIL IN TREATING TYPE IIA PATIENTS WITH ELEVATIONS OF LDL-CHOLESTEROL ONLY IS NOT LIKELY TO OUTWEIGH THE RISKS. GEMFIBROZIL IS ALSO NOT INDICATED FOR THE TREATMENT OF PATIENTS WITH LOW HDL-CHOLESTEROL AS THEIR ONLY LIPID ABNORMALITY.
Table I).
GEMFIBROZIL CONTRAINDICATIONS
WARNINGS).
PRECAUTIONS).
WARNINGS
CLINICAL PHARMACOLOGY). Noncoronary heart disease related mortality showed an excess in the group originally randomized to gemfibrozil primarily due to cancer deaths observed during the open-label extension.
CLINICAL PHARMACOLOGY).
A comparative carcinogenicity study was also done in rats comparing three drugs in this class: fenofibrate (10 and 60 mg/kg; 0.3 and 1.6 times the human dose, respectively), clofibrate (40 mg/kg; 1.6 times the human dose), and gemfibrozil (250 mg/kg; 1.7 times the human dose). Pancreatic acinar adenomas were increased in males and females on fenofibrate; hepatocellular carcinoma and pancreatic acinar adenomas were increased in males and hepatic neoplastic nodules in females treated with clofibrate; hepatic neoplastic nodules were increased in males and females treated with gemfibrozil while testicular interstitial cell (leydig cell) tumors were increased in males on all three drugs.
INDICATIONS AND USAGEsection. If a significant serum lipid response is not obtained, gemfibrozil should be discontinued.
PRECAUTIONS, Drug Interactions). The use of fibrates alone, including gemfibrozil may occasionally be associated with myositis. Patients receiving gemfibrozil and complaining of muscle pain, tenderness, or weakness should have prompt medical evaluation for myositis, including serum creatine-kinase level determination. If myositis is suspected or diagnosed, gemfibrozil therapy should be withdrawn.
PRECAUTIONS
Initial TherapyContinued Therapy
Drug Interactions
WARNINGS). There is no assurance that periodic monitoring of creatine kinase will prevent the occurrence of severe myopathy and kidney damage.
CONTRAINDICATIONS).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
Nursing Mothers
Hematologic Changes
Liver Function
Kidney Function
Pediatric Use
GEMFIBROZIL ADVERSE REACTIONS
GEMFIBROZIL
(N = 2046) PLACEBO
(N = 2035) Frequency in percent of subjects
Gallbladder surgerywas performed in 0.9% of gemfibrozil and 0.5% of placebo subjects in the primary prevention component, a 64% excess, which is not statistically different from the excess of gallbladder surgery observed in the clofibrate group compared to the placebo group of the WHO study. Gallbladder surgery was also performed more frequently in the gemfibrozil group compared to the placebo group (1.9% versus 0.3%, p = 0.07) in the secondary prevention component. A statistically significant increase in appendectomy in the gemfibrozil group was seen also in the secondary prevention component (6 on gemfibrozil versus 0 on placebo, p = 0.014).
WARNINGS), and to ABNORMAL LIVER FUNCTION TESTS and HEMATOLOGIC CHANGES (seePRECAUTIONS).
CAUSAL RELATIONSHIP PROBABLE CAUSAL RELATIONSHIP NOT ESTABLISHED WARNINGSandPRECAUTIONS, Drug Interactions) Additional adverse reactions that have been reported include cholecystitis and cholelithiasis(seeWARNINGS).
DOSAGE & ADMINISTRATION
CLINICAL PHARMACOLOGY).OVERDOSAGE
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

GemfibrozilGEMFIBROZIL TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!