Gemfibrozil
FULL PRESCRIBING INFORMATION: CONTENTS*
- GEMFIBROZIL DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- GEMFIBROZIL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- GEMFIBROZIL ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- OVERDOSAGE
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
GEMFIBROZIL DESCRIPTION
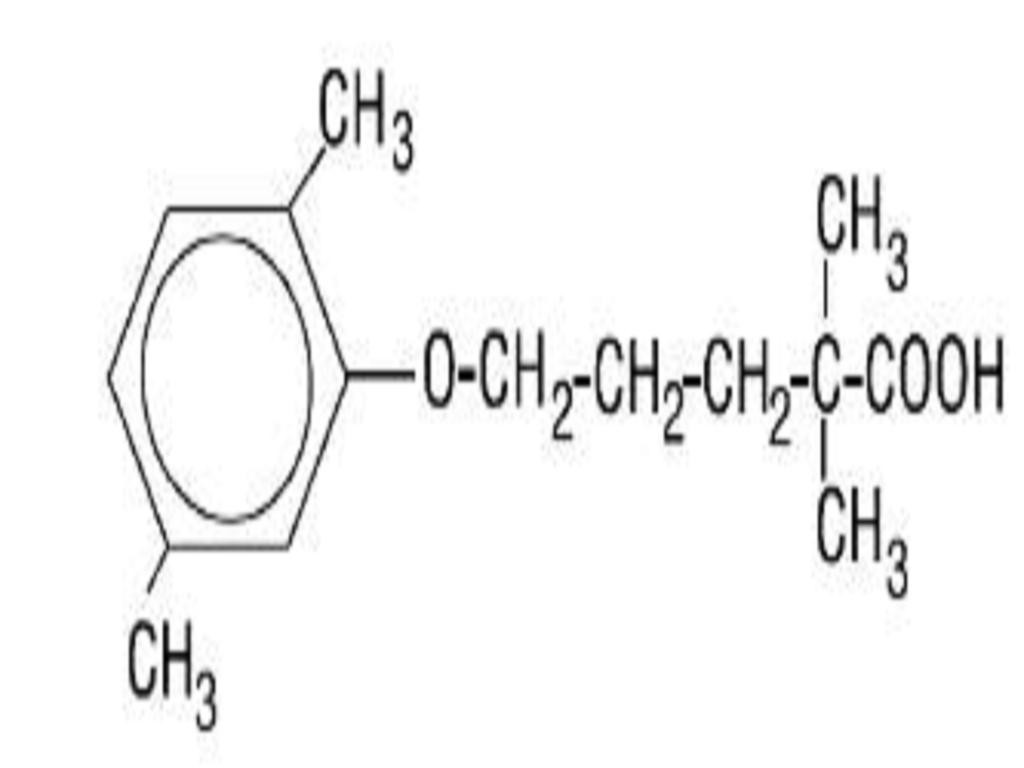
CLINICAL PHARMACOLOGY
All Patients
Group:PDropPNPGGDropGNGGN=215N=494N=1283N=221N=574N=1207
EventGemfibrozil at Study StartPlacebo at Study StartGemfibrozil:Placebo Hazard Ratio2CI Hazard Ratio3
DOSAGE AND ADMINISTRATION
PRECAUTIONS
INDICATIONS & USAGE
WARNINGSPRECAUTIONSCLINICAL PHARMACOLOGY
GEMFIBROZIL CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
WARNINGS
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
INDICATIONS AND USAGE
PRECAUTIONSDrug Interactions
PRECAUTIONS
1. Initial Therapy -2. Continued Therapy -
3. Drug Interactions -
WARNINGS
CONTRAINDICATIONS
4. Carcinogenesis, Mutagenesis, Impairment of Fertility -
5. Pregnancy Category C -
6. Nursing Mothers -
7. Hematologic Changes -
8. Liver Function -
9. Kidney Function -
10. Pediatric Use -
GEMFIBROZIL ADVERSE REACTIONS
ADVERSE REACTIONSGEMFIBROZIL (N = 2046)PLACEBO (N = 2035)Frequency in percent of subjects
WARNINGSPRECAUTIONS
CAUSAL RELATIONSHIP PROBABLECAUSAL RELATIONSHIP NOT ESTABLISHEDWARNINGSDrug InteractionsPRECAUTIONSWARNINGS
DOSAGE & ADMINISTRATION
CLINICAL PHARMACOLOGYOVERDOSAGE
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

GemfibrozilGemfibrozil TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!