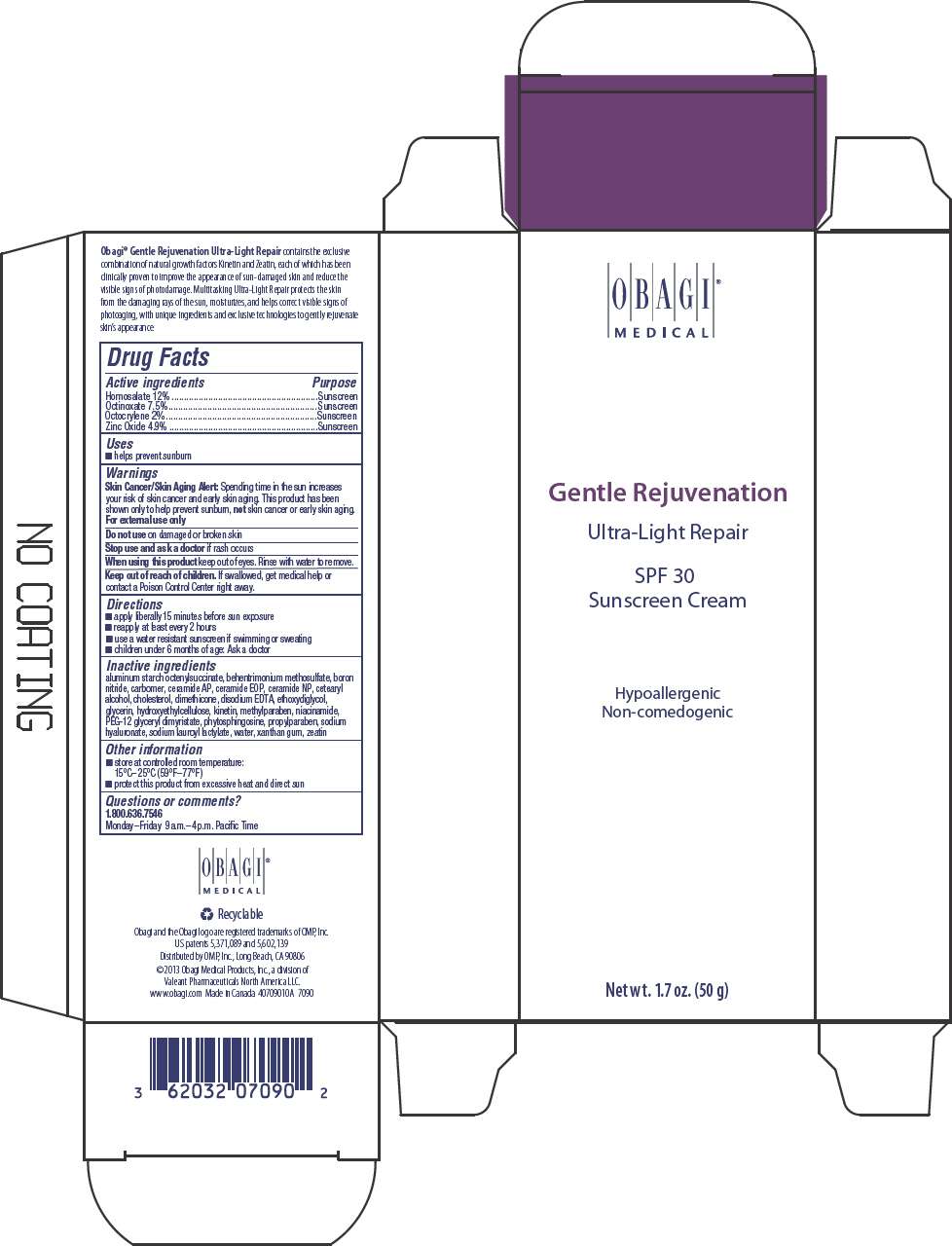
GENTLE REJUVENATION ULTRA LIGHT REPAIR SPF 30
Obagi Medical Products, Inc., a division of Valeant Pharmaceutcals North America LLC.
GENTLE REJUVENATION ULTRA LIGHT REPAIR SPF 30 SUNSCREEN CREAM
FULL PRESCRIBING INFORMATION: CONTENTS*
- GENTLE REJUVENATION ULTRA LIGHT REPAIR SPF 30 Uses
- Warnings
- Directions
- Inactive ingredients
- GENTLE REJUVENATION ULTRA LIGHT REPAIR SPF 30 Other information
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 50 g Tube Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active ingredients | Purpose |
|---|---|
| Homosalate 12% | Sunscreen |
| Octinoxate 7.5% | Sunscreen |
| Octocrylene 2% | Sunscreen |
| Zinc Oxide 4.9% | Sunscreen |
GENTLE REJUVENATION ULTRA LIGHT REPAIR SPF 30 Uses
- helps prevent sunburn
Warnings
Skin Cancer/Skin Aging Alert
Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
For external use only
Do not use on damaged or broken skin
Stop use and ask a doctor if rash occurs
When using this product keep out of eyes. Rinse with water to remove.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- children under 6 months of age: Ask a doctor
Inactive ingredients
aluminum starch octenylsuccinate, behentrimonium methosulfate, boron nitride, carbomer, ceramide AP, ceramide EOP, ceramide NP, cetearyl alcohol, cholesterol, dimethicone, disodium EDTA, ethoxydiglycol, glycerin, hydroxyethylcellulose, kinetin, methylparaben, niacinamide, PEG-12 glyceryl dimyristate, phytosphingosine, propylparaben, sodium hyaluronate, sodium lauroyl lactylate, water, xanthan gum, zeatin
GENTLE REJUVENATION ULTRA LIGHT REPAIR SPF 30 Other information
- store at controlled room temperature:
15°C–25°C (59°F–77°F) - protect this product from excessive heat and direct sun
Questions or comments?
1.800.636.7546
Monday–Friday 9 a.m.–4 p.m. Pacific Time
Distributed by OMP, Inc., Long Beach, CA 90806
PRINCIPAL DISPLAY PANEL - 50 g Tube Carton
OBAGI®
MEDICAL
Gentle Rejuvenation
Ultra-Light Repair
SPF 30
Sunscreen Cream
Hypoallergenic
Non-comedogenic
Net wt. 1.7 oz. (50 g)
GENTLE REJUVENATION ULTRA LIGHT REPAIR SPF 30HOMOSALATE, OCTINOXATE, OCTOCRYLENE, and ZINC OXIDE CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||