Gilenya
Novartis Pharmaceuticals Corporation
HIGHLIGHTS OF PRESCRIBING INFORMATION RECENT MAJOR CHANGESDosage and Administration (2) 4/2014Warnings and Precautions: (5.2, 5.4, 5.8) 4/2014INDICATIONS AND USAGEGILENYA is a sphingosine 1-phosphate receptor modulator indicated for the treatment of patients with relapsing forms of multiple sclerosis (MS) to reduce the frequency of clinical exacerbations and to delay the accumulation of physical disability. (1)DOSAGE AND ADMINISTRATION Recommended dose: 0.5 mg orally once daily, with or without food (2) First Dose Monitoring: Observe all patients for bradycardia for at least 6 hours after first dose with hourly pulse and blood pressure measurement. Obtain electrocardiogram (ECG) prior to dosing and at end of observation period. Patients who develop heart rate placebo): Headache, influenza, diarrhea, back pain, liver transaminase elevations, and cough. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Ketoconazole: Closely monitor during concomitant use with systemic ketoconazole (7, 12.3) Vaccines: Avoid live attenuated vaccines during, and for 2 months after stopping GILENYA treatment. (5.2, 7) USE IN SPECIFIC POPULATIONS Pregnancy: Based on animal data, may cause fetal harm. (8.1) Hepatic impairment: Closely monitor patients with severe hepatic impairment. (5.6, 8.6, 12.3)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 GILENYA INDICATIONS AND USAGE
- 2 GILENYA DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 GILENYA CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 GILENYA ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 GILENYA DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
GILENYA is indicated for the treatment of patients with relapsing forms of multiple sclerosis (MS) to reduce the frequency of clinical exacerbations and to delay the accumulation of physical disability.
2 DOSAGE AND ADMINISTRATION
Recommended Dose
The recommended dose of GILENYA is 0.5 mg orally once daily. Fingolimod doses higher than 0.5 mg are associated with a greater incidence of adverse reactions without additional benefit. GILENYA can be taken with or without food.
First Dose Monitoring
Initiation of GILENYA treatment results in a decrease in heart rate [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.2)]. After the first dose of GILENYA, the heart rate decrease starts within an hour and the Day 1 nadir generally occurs within approximately 6 hours, although the nadir can be observed up to 24 hours after the first dose in some patients.
The first dose of GILENYA should be administered in a setting in which resources to appropriately manage symptomatic bradycardia are available. In order to assess patient response to the first dose of fingolimod, observe all patients for 6 hours for signs and symptoms of bradycardia with hourly pulse and blood pressure measurement. Obtain in all patients an electrocardiogram (ECG) prior to dosing, and at the end of the observation period.
Additional observation should be instituted until the finding has resolved in the following situations:
- The heart rate 6 hours postdose is <45 bpm
- The heart rate 6 hours postdose is at the lowest value postdose (suggesting that the maximum pharmacodynamic effect on the heart may not have occurred)
- The ECG 6-hours postdose shows new onset second degree or higher atrioventricular (AV) block
Should postdose symptomatic bradycardia occur, initiate appropriate management, begin continuous ECG monitoring, and continue observation until the symptoms have resolved.
Should a patient require pharmacologic intervention for symptomatic bradycardia, continuous overnight ECG monitoring in a medical facility should be instituted, and the first dose monitoring strategy should be repeated after the second dose of GILENYA.
Patients with some preexisting conditions (e.g., ischemic heart disease, history of myocardial infarction, congestive heart failure, history of cardiac arrest, cerebrovascular disease, uncontrolled hypertension, history of symptomatic bradycardia, history of recurrent syncope, severe untreated sleep apnea, AV block, sinoatrial heart block) may poorly tolerate the GILENYA-induced bradycardia, or experience serious rhythm disturbances after the first dose of GILENYA. Prior to treatment with GILENYA, these patients should have a cardiac evaluation by a physician appropriately trained to conduct such evaluation, and, if treated with GILENYA, should be monitored overnight with continuous ECG in a medical facility after the first dose. GILENYA is contraindicated in patients who in the last 6 months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization or Class III/IV heart failure) [see Contraindications (4)].
Since initiation of GILENYA treatment results in decreased heart rate and may prolong the QT interval, patients with a prolonged QTc interval (>450 msec males, >470 msec females) before dosing or during 6 hour observation, or at additional risk for QT prolongation (e.g., hypokalemia, hypomagnesemia, congenital long-QT syndrome), or on concurrent therapy with QT prolonging drugs with a known risk of torsades de pointes (e.g., citalopram, chlorpromazine, haloperidol, methadone, erythromycin) should be monitored overnight with continuous ECG in a medical facility [see Drug Interactions (7)].
Experience with GILENYA is limited in patients receiving concurrent therapy with drugs that slow heart rate or atrioventricular conduction (e.g., beta blockers, heart-rate lowering calcium channel blockers such as diltiazem or verapamil, or digoxin). Because the initiation of GILENYA treatment is also associated with slowing of the heart rate, concomitant use of these drugs during GILENYA initiation may be associated with severe bradycardia or heart block. The possibility to switch to drugs that do not slow the heart rate or atrioventricular conduction should be evaluated by the physician prescribing these drugs before initiating GILENYA. Patients who cannot switch should have overnight continuous ECG monitoring after the first dose [see Drug Interactions (7)].
Clinical data indicate effects of GILENYA on heart rate are maximal after the first dose although milder effects on heart rate may persist for, on average, 2 to 4 weeks after initiation of therapy at which time heart rate generally returns to baseline. Physicians should continue to be alert to patient reports of cardiac symptoms.
Reinitiation of Therapy Following Discontinuation
If GILENYA therapy is discontinued for more than 14 days, after the first month of treatment, the effects on heart rate and AV conduction may recur on reintroduction of GILENYA treatment and the same precautions (first dose monitoring) as for initial dosing should apply. Within the first 2 weeks of treatment, first dose procedures are recommended after interruption of 1 day or more; during weeks 3 and 4 of treatment first dose procedures are recommended after treatment interruption of more than 7 days.
3 DOSAGE FORMS AND STRENGTHS
GILENYA is available as 0.5 mg hard capsules with a white opaque body and bright yellow cap imprinted with “FTY 0.5 mg” on the cap and 2 radial bands imprinted on the capsule body with yellow ink.
4 CONTRAINDICATIONS
Patients who in the last 6 months experienced myocardial infarction, unstable angina, stroke, TIA, decompensated heart failure requiring hospitalization or Class III/IV heart failure
History or presence of Mobitz Type II second-degree or third-degree atrioventricular (AV) block or sick sinus syndrome, unless patient has a functioning pacemaker
Baseline QTc interval ≥500 msec
Treatment with Class Ia or Class III anti-arrhythmic drugs
5 WARNINGS AND PRECAUTIONS
5.1 Bradyarrhythmia and Atrioventricular Blocks
Because of a risk for bradyarrhythmia and atrioventricular (AV) blocks, patients should be monitored during GILENYA treatment initiation [see Dosage and Administration (2)].
Reduction in Heart Rate
After the first dose of GILENYA, the heart rate decrease starts within an hour. On Day 1, the maximal decline in heart rate generally occurs within 6 hours and recovers, although not to baseline levels, by 8- 10 hours postdose. Because of physiological diurnal variation, there is a second period of heart rate decrease within 24 hours after the first dose. In some patients, heart rate decrease during the second period is more pronounced than the decrease observed in the first 6 hours. Heart rates below 40 beats per minute were rarely observed. Adverse reactions of symptomatic bradycardia following the first dose were reported in 0.5% of patients receiving GILENYA 0.5 mg, but in no patient on placebo. Patients who experienced bradycardia were generally asymptomatic, but some patients experienced hypotension, dizziness, fatigue, palpitations, and chest pain that usually resolved within the first 24 hours on treatment.
Following the second dose, a further decrease in heart rate may occur when compared to the heart rate prior to the second dose, but this change is of a smaller magnitude than that observed following the first dose. With continued dosing, the heart rate returns to baseline within 1 month of chronic treatment.
Atrioventricular Blocks
Initiation of GILENYA treatment has resulted in transient AV conduction delays. In controlled clinical trials, adverse reactions of first-degree AV block (prolonged PR interval on ECG) following the first dose were reported in 0.1% of patients receiving GILENYA 0.5 mg, but in no patient on placebo. Second-degree AV blocks following the first dose were also identified in 0.1% of patients receiving GILENYA 0.5 mg, but in no patient on placebo. In a study of 698 patients with available 24-hour Holter monitoring data after their first dose (N=351 on GILENYA 0.5 mg and N=347 on placebo), second-degree AV blocks, Mobitz types I (Wenckebach) and/or II, were reported in 3.7% (N=13) of patients receiving GILENYA 0.5 mg and 2% (N=7) of patients on placebo. The conduction abnormalities were usually transient and asymptomatic, and resolved within the first 24 hours on treatment, but they occasionally required treatment with atropine or isoproterenol.
Postmarketing Experience
In the postmarketing setting, third-degree AV block and AV block with junctional escape have been observed during the first-dose 6-hour observation period with GILENYA. Isolated delayed onset events, including transient asystole and unexplained death, have occurred within 24 hours of the first dose. These events were confounded by concomitant medications and/or preexisting disease, and the relationship to GILENYA is uncertain. Cases of syncope were also reported after the first dose of GILENYA.
5.2 Infections
Risk of Infections
GILENYA causes a dose-dependent reduction in peripheral lymphocyte count to 20%–30% of baseline values because of reversible sequestration of lymphocytes in lymphoid tissues. GILENYA may therefore increase the risk of infections, some serious in nature [see Clinical Pharmacology (12.2)].
Before initiating treatment with GILENYA, a recent CBC (i.e., within 6 months) should be available. Consider suspending treatment with GILENYA if a patient develops a serious infection, and reassess the benefits and risks prior to reinitiation of therapy. Because the elimination of fingolimod after discontinuation may take up to 2 months, continue monitoring for infections throughout this period. Instruct patients receiving GILENYA to report symptoms of infections to a physician. Patients with active acute or chronic infections should not start treatment until the infection(s) is resolved.
Two patients died of herpetic infections during GILENYA controlled studies in the premarketing database (1 disseminated primary herpes zoster and 1 herpes simplex encephalitis). In both cases, the patients were receiving a fingolimod dose (1.25 mg) higher than recommended for the treatment of MS (0.5 mg), and had received high-dose corticosteroid therapy for suspected MS relapse. No deaths due to viral infections occurred in patients treated with GILENYA 0.5 mg in the premarketing database.
In MS controlled studies, the overall rate of infections (72%) and serious infections (2%) with GILENYA 0.5 mg was similar to placebo. However, bronchitis and, to a lesser extent, pneumonia were more common in GILENYA-treated patients.
Prior and Concomitant Treatment with Antineoplastic, Immunosuppressive, or Immune-Modulating Therapies
GILENYA has not been administered concomitantly with antineoplastic, immunosuppressive, or immune-modulating therapies used for treatment of MS. Concomitant use of GILENYA with any of these therapies would be expected to increase the risk of immunosuppression [see Drug Interactions (7)].
When switching from other immunosuppressive medications, the duration and mode of action of such substances must be considered when initiating Gilenya to avoid additive immunosuppressive effects.
Varicella Zoster Virus Antibody Testing/Vaccination
As for any immune-modulating drug, before initiating GILENYA therapy, patients without a history of chickenpox or without vaccination against varicella zoster virus (VZV) should be tested for antibodies to VZV. VZV vaccination of antibody-negative patients should be considered prior to commencing treatment with GILENYA, following which initiation of treatment with GILENYA should be postponed for 1 month to allow the full effect of vaccination to occur.
5.3 Macular Edema
In patients receiving GILENYA 0.5 mg, macular edema occurred in 0.4% of patients. An adequate ophthalmologic evaluation should be performed at baseline and 3-4 months after treatment initiation. If patients report visual disturbances at any time while on GILENYA therapy, additional ophthalmologic evaluation should be undertaken.
In MS controlled studies involving 1204 patients treated with GILENYA 0.5 mg and 861 patients treated with placebo, macular edema with or without visual symptoms was reported in 0.4% of patients treated with GILENYA 0.5 mg and 0.1% of patients treated with placebo; it occurred predominantly in the first 3-4 months of therapy. Some patients presented with blurred vision or decreased visual acuity, but others were asymptomatic and diagnosed on routine ophthalmologic examination. Macular edema generally improved or resolved with or without treatment after drug discontinuation, but some patients had residual visual acuity loss even after resolution of macular edema.
Continuation of GILENYA in patients who develop macular edema has not been evaluated. A decision on whether or not to discontinue GILENYA therapy should include an assessment of the potential benefits and risks for the individual patient. The risk of recurrence after rechallenge has not been evaluated.
Macular Edema in Patients with History of Uveitis or Diabetes Mellitus
Patients with a history of uveitis and patients with diabetes mellitus are at increased risk of macular edema during GILENYA therapy. The incidence of macular edema is also increased in MS patients with a history of uveitis. The rate was approximately 20% in patients with a history of uveitis versus 0.6% in those without a history of uveitis, in the combined experience with all doses of fingolimod. MS patients with diabetes mellitus or a history of uveitis should undergo an ophthalmologic evaluation prior to initiating GILENYA therapy and have regular follow-up ophthalmologic evaluations while receiving GILENYA therapy. GILENYA has not been tested in MS patients with diabetes mellitus.
5.4 Posterior Reversible Encephalopathy Syndrome
There have been rare cases of posterior reversible encephalopathy syndrome (PRES) reported in patients receiving Gilenya. Symptoms reported included sudden onset of severe headache, altered mental status, visual disturbances, and seizure. Symptoms of PRES are usually reversible but may evolve into ischemic stroke or cerebral hemorrhage. Delay in diagnosis and treatment may lead to permanent neurological sequelae. If PRES is suspected, GILENYA should be discontinued.
5.5 Respiratory Effects
Dose-dependent reductions in forced expiratory volume over 1 second (FEV1) and diffusion lung capacity for carbon monoxide (DLCO) were observed in patients treated with GILENYA as early as 1 month after treatment initiation. At Month 24, the reduction from baseline in the percent of predicted values for FEV1 was 3.1% for GILENYA 0.5 mg and 2% for placebo. For DLCO, the reductions from baseline in percent of predicted values at Month 24 were 3.8% for GILENYA 0.5 mg and 2.7% for placebo. The changes in FEV1 appear to be reversible after treatment discontinuation. There is insufficient information to determine the reversibility of the decrease of DLCO after drug discontinuation. In MS controlled trials, dyspnea was reported in 5% of patients receiving GILENYA 0.5 mg and 4% of patients receiving placebo. Several patients discontinued GILENYA because of unexplained dyspnea during the extension (uncontrolled) studies. GILENYA has not been tested in MS patients with compromised respiratory function.
Spirometric evaluation of respiratory function and evaluation of DLCO should be performed during therapy with GILENYA if clinically indicated.
5.6 Liver Injury
Elevations of liver enzymes may occur in patients receiving GILENYA. Recent (i.e., within last 6 months) transaminase and bilirubin levels should be available before initiation of GILENYA therapy.
During clinical trials, 3-fold the upper limit of normal (ULN) or greater elevation in liver transaminases occurred in 8% of patients treated with GILENYA 0.5 mg, as compared to 2% of patients on placebo. Elevations 5-fold the ULN occurred in 2% of patients on GILENYA and 1% of patients on placebo. In clinical trials, GILENYA was discontinued if the elevation exceeded 5 times the ULN. Recurrence of liver transaminase elevations occurred with rechallenge in some patients, supporting a relationship to drug. The majority of elevations occurred within 6-9 months. Serum transaminase levels returned to normal within approximately 2 months after discontinuation of GILENYA.
Liver enzymes should be monitored in patients who develop symptoms suggestive of hepatic dysfunction, such as unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine. GILENYA should be discontinued if significant liver injury is confirmed. Patients with preexisting liver disease may be at increased risk of developing elevated liver enzymes when taking GILENYA.
Because GILENYA exposure is doubled in patients with severe hepatic impairment, these patients should be closely monitored, as the risk of adverse reactions is greater [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
5.7 Fetal Risk
Based on animal studies, GILENYA may cause fetal harm. Because it takes approximately 2 months to eliminate GILENYA from the body, women of childbearing potential should use effective contraception to avoid pregnancy during and for 2 months after stopping GILENYA treatment.
5.8 Blood Pressure Effects
In MS clinical trials, patients treated with GILENYA 0.5 mg had an average increase over placebo of approximately 3 mmHg in systolic pressure, and approximately 2 mmHg in diastolic pressure, first detected after approximately 1 month of treatment initiation, and persisting with continued treatment. Hypertension was reported as an adverse reaction in 8% of patients on GILENYA 0.5 mg and in 4% of patients on placebo. Blood pressure should be monitored during treatment with GILENYA.
5.9 Immune System Effects Following GILENYA Discontinuation
Fingolimod remains in the blood and has pharmacodynamic effects, including decreased lymphocyte counts, for up to 2 months following the last dose of GILENYA. Lymphocyte counts generally return to the normal range within 1-2 months of stopping therapy [see Clinical Pharmacology (12.2)]. Because of the continuing pharmacodynamic effects of fingolimod, initiating other drugs during this period warrants the same considerations needed for concomitant administration (e.g., risk of additive immunosuppressant effects) [see Drug Interactions (7)].
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in labeling:
- Bradyarrhythmia and Atrioventricular Blocks [see Warnings and Precautions (5.1)]
- Infections [see Warnings and Precautions (5.2)]
- Macular Edema [see Warnings and Precautions (5.3)]
- Posterior Reversible Encephalopathy Syndrome [see Warnings and Precautions (5.4)]
- Respiratory Effects [see Warnings and Precautions (5.5)]
- Liver Injury [see Warnings and Precautions (5.6)]
The most frequent adverse reactions (incidence ≥10% and >placebo) for GILENYA 0.5 mg were headache, influenza, diarrhea, back pain, liver enzyme elevations, and cough. The only adverse event leading to treatment interruption reported at an incidence >1% for GILENYA 0.5 mg was serum transaminase elevations (3.8%).
6.1 Clinical Trials Experience
A total of 1703 patients on GILENYA (0.5 or 1.25 mg once daily) constituted the safety population in the 2 controlled studies in patients with relapsing-remitting MS (RRMS) [see Clinical Studies (14)].
Study 1 was a 2-year placebo-controlled clinical study in 1272 MS patients treated with GILENYA 0.5 mg (N=425), GILENYA 1.25 mg (N=429), or placebo (N=418).
|
Primary System Organ Class
Preferred Term |
GILENYA 0.5 mg
N=425 % |
Placebo
N=418 % |
| Infections | ||
| Influenza viral infections | 13 | 10 |
| Herpes viral infections | 9 | 8 |
| Bronchitis | 8 | 4 |
| Sinusitis | 7 | 5 |
| Gastroenteritis | 5 | 3 |
| Tinea infections | 4 | 1 |
| Cardiac Disorders | ||
| Bradycardia | 4 | 1 |
| Nervous system disorders | ||
| Headache | 25 | 23 |
| Dizziness | 7 | 6 |
| Paresthesia | 5 | 4 |
| Migraine | 5 | 1 |
| Gastrointestinal disorders | ||
| Diarrhea | 12 | 7 |
| General disorders and administration site conditions | ||
| Asthenia | 3 | 1 |
| Musculoskeletal and connective tissue disorders | ||
| Back pain | 12 | 7 |
| Skin and subcutaneous tissue disorders | ||
| Alopecia | 4 | 2 |
| Eczema | 3 | 2 |
| Pruritus | 3 | 1 |
| Investigations | ||
| ALT/AST increased | 14 | 5 |
| GGT increased | 5 | 1 |
| Weight decreased | 5 | 3 |
| Blood triglycerides increased | 3 | 1 |
| Respiratory, thoracic, and mediastinal disorders | ||
| Cough | 10 | 8 |
| Dyspnea | 8 | 5 |
| Psychiatric disorders | ||
| Depression | 8 | 7 |
| Eye disorders | ||
| Vision blurred | 4 | 1 |
| Eye pain | 3 | 1 |
| Vascular disorders | ||
| Hypertension | 6 | 4 |
| Blood and lymphatic system disorders | ||
| Lymphopenia | 4 | 1 |
| Leukopenia | 3 | <1 |
Adverse reactions in Study 2, a 1-year active-controlled (versus interferon beta-1a, n=431) study including 849 patients with MS treated with fingolimod, were generally similar to those in Study 1.
Vascular Events
Vascular events, including ischemic and hemorrhagic strokes, and peripheral arterial occlusive disease were reported in premarketing clinical trials in patients who received GILENYA doses (1.25-5 mg) higher than recommended for use in MS. Similar events have been reported with GILENYA 0.5 mg in the postmarketing setting although a causal relationship has not been established.
Lymphomas
Cases of lymphoma (cutaneous T-cell lymphoproliferative disorders or diffuse B-cell lymphoma) were reported in premarketing clinical trials in MS patients receiving GILENYA at, or above, the recommended dose of 0.5 mg. Based on the small number of cases and short duration of exposure, the relationship to GILENYA remains uncertain.
7 DRUG INTERACTIONS
QT Prolonging Drugs
GILENYA has not been studied in patients treated with drugs that prolong the QT interval. Drugs that prolong the QT interval have been associated with cases of torsades de pointes in patients with bradycardia. Since initiation of GILENYA treatment results in decreased heart rate and may prolong the QT interval, patients on QT prolonging drugs with a known risk of torsades de pointes (e.g., citalopram, chlorpromazine, haloperidol, methadone, erythromycin) should be monitored overnight with continuous ECG in a medical facility [see Dosage and Administration (2) and Warnings and Precautions (5.1)].
Ketoconazole
The blood levels of fingolimod and fingolimod-phosphate are increased by 1.7-fold when used concomitantly with ketoconazole. Patients who use GILENYA and systemic ketoconazole concomitantly should be closely monitored, as the risk of adverse reactions is greater.
Vaccines
GILENYA reduces the immune response to vaccination. Vaccination may be less effective during and for up to 2 months after discontinuation of treatment with GILENYA [see Clinical Pharmacology (12.2)]. The use of live attenuated vaccines should be avoided during and for 2 months after treatment with GILENYA because of the risk of infection.
Antineoplastic, Immunosuppressive, or Immunomodulating Therapies
Antineoplastic, immunosuppressive, or immune-modulating therapies are expected to increase the risk of immunosuppression. Use caution when switching patients from long-acting therapies with immune effects such as natalizumab or mitoxantrone [see Warnings and Precautions (5.2)].
Drugs That Slow Heart Rate or Atrioventricular Conduction (e.g., beta blockers or diltiazem)
Experience with GILENYA in patients receiving concurrent therapy with drugs that slow the heart rate or atrioventricular conduction (e.g., beta blockers, digoxin, or heart rate-slowing calcium channel blockers such as diltiazem or verapamil) is limited. Because initiation of GILENYA treatment may result in an additional decrease in heart rate, concomitant use of these drugs during GILENYA initiation may be associated with severe bradycardia or heart block. Seek advice from the prescribing physician regarding the possibility to switch to drugs that do not slow the heart rate or atrioventricular conduction before initiating GILENYA. Patients who cannot switch, should have overnight continuous ECG monitoring after the first dose [see Dosage and Administration (2) and Warnings and Precautions (5.1)].
Laboratory Test Interaction
Because GILENYA reduces blood lymphocyte counts via redistribution in secondary lymphoid organs, peripheral blood lymphocyte counts cannot be utilized to evaluate the lymphocyte subset status of a patient treated with GILENYA. A recent CBC should be available before initiating treatment with GILENYA.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women. In oral studies conducted in rats and rabbits, fingolimod demonstrated developmental toxicity, including teratogenicity (rats) and embryolethality, when given to pregnant animals. In rats, the highest no-effect dose was less than the recommended human dose (RHD) of 0.5 mg/day on a body surface area (mg/m2) basis. The most common fetal visceral malformations in rats included persistent truncus arteriosus and ventricular septal defect. The receptor affected by fingolimod (sphingosine 1-phosphate receptor) is known to be involved in vascular formation during embryogenesis. Because it takes approximately 2 months to eliminate fingolimod from the body, potential risks to the fetus may persist after treatment ends [see Warnings and Precautions (5.7, 5.9)]. GILENYA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Registry
A pregnancy registry has been established to collect information about the effect of GILENYA use during pregnancy. Physicians are encouraged to enroll pregnant patients, or pregnant women may register themselves in the GILENYA pregnancy registry by calling Outcome at 1-877-598-7237, sending an email to gpr@outcome.com or visiting www.gilenyapregnancyregistry.com.
Animal Data
When fingolimod was orally administered to pregnant rats during the period of organogenesis (0, 0.03, 0.1, and 0.3 mg/kg/day or 0, 1, 3, and 10 mg/kg/day), increased incidences of fetal malformations and embryo-fetal deaths were observed at all but the lowest dose tested (0.03 mg/kg/day), which is less than the RHD on a mg/m2 basis. Oral administration to pregnant rabbits during organogenesis (0, 0.5, 1.5, and 5 mg/kg/day) resulted in increased incidences of embryo-fetal mortality and fetal growth retardation at the mid and high doses. The no-effect dose for these effects in rabbits (0.5 mg/kg/day) is approximately 20 times the RHD on a mg/m2 basis.
When fingolimod was orally administered to female rats during pregnancy and lactation (0, 0.05, 0.15, and 0.5 mg/kg/day), pup survival was decreased at all doses and a neurobehavioral (learning) deficit was seen in offspring at the high dose. The low-effect dose of 0.05 mg/kg/day is similar to the RHD on a mg/m2 basis.
8.2 Labor and Delivery
The effects of GILENYA on labor and delivery are unknown.
8.3 Nursing Mothers
Fingolimod is excreted in the milk of treated rats. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from GILENYA, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and effectiveness of GILENYA in pediatric patients with MS below the age of 18 years have not been established.
In a study in which fingolimod (0.3, 1.5, or 7.5 mg/kg/day) was orally administered to young rats from weaning through sexual maturity, changes in bone mineral density and persistent neurobehavioral impairment (altered auditory startle) were observed at all doses. Delayed sexual maturation was noted in females at the highest dose tested and in males at all doses. The bone changes observed in fingolimod-treated juvenile rats are consistent with a reported role of S1P in the regulation of bone mineral homeostasis.
When fingolimod (0.5 or 5 mg/kg/day) was orally administered to rats from the neonatal period through sexual maturity, a marked decrease in T-cell dependent antibody response was observed at both doses. This effect had not fully recovered by 6-8 weeks after the end of treatment.
8.5 Geriatric Use
Clinical MS studies of GILENYA did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently than younger patients. GILENYA should be used with caution in patients aged 65 years and over, reflecting the greater frequency of decreased hepatic, or renal, function and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
Because fingolimod, but not fingolimod-phosphate, exposure is doubled in patients with severe hepatic impairment, patients with severe hepatic impairment should be closely monitored, as the risk of adverse reactions may be greater [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)].
No dose adjustment is needed in patients with mild or moderate hepatic impairment.
8.7 Renal Impairment
The blood level of some GILENYA metabolites is increased (up to 13-fold) in patients with severe renal impairment [see Clinical Pharmacology (12.3)]. The toxicity of these metabolites has not been fully explored. The blood level of these metabolites has not been assessed in patients with mild or moderate renal impairment.
10 OVERDOSAGE
GILENYA can induce bradycardia as well as AV conduction blocks (including complete AV block). The decline in heart rate usually starts within 1 hour of the first dose and is maximal within 6 hours in most patients [see Warnings and Precautions (5.1)]. In case of GILENYA overdosage, observe patients overnight with continuous ECG monitoring in a medical facility, and obtain regular measurements of blood pressure [see Dosage and Administration (2)].
Neither dialysis nor plasma exchange results in removal of fingolimod from the body.
11 DESCRIPTION
Fingolimod is a sphingosine 1-phosphate receptor modulator.
Chemically, fingolimod is 2-amino-2-[2-(4-octylphenyl)ethyl]propan-1,3-diol hydrochloride. Its structure is shown below:
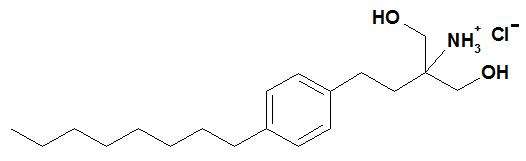
Fingolimod hydrochloride is a white to practically white powder that is freely soluble in water and alcohol and soluble in propylene glycol. It has a molecular weight of 343.93.
GILENYA is provided as 0.5 mg hard gelatin capsules for oral use. Each capsule contains 0.56 mg of fingolimod hydrochloride, equivalent to 0.5 mg of fingolimod.
Each GILENYA 0.5 mg capsule contains the following inactive ingredients: gelatin, magnesium stearate, mannitol, titanium dioxide, yellow iron oxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fingolimod is metabolized by sphingosine kinase to the active metabolite, fingolimod-phosphate. Fingolimod-phosphate is a sphingosine 1-phosphate receptor modulator, and binds with high affinity to sphingosine 1-phosphate receptors 1, 3, 4, and 5. Fingolimod-phosphate blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. The mechanism by which fingolimod exerts therapeutic effects in multiple sclerosis is unknown, but may involve reduction of lymphocyte migration into the central nervous system.
12.2 Pharmacodynamics
Heart Rate and Rhythm
Fingolimod causes a transient reduction in heart rate and AV conduction at treatment initiation [see Warnings and Precautions (5.1)].
Heart rate progressively increases after the first day, returning to baseline values within 1 month of the start of chronic treatment.
Autonomic responses of the heart, including diurnal variation of heart rate and response to exercise, are not affected by fingolimod treatment.
Fingolimod treatment is not associated with a decrease in cardiac output.
Potential to Prolong the QT Interval
In a thorough QT interval study of doses of 1.25 or 2.5 mg fingolimod at steady-state, when a negative chronotropic effect of fingolimod was still present, fingolimod treatment resulted in a prolongation of QTc, with the upper bound of the 90% confidence interval (CI) of 14.0 msec. There is no consistent signal of increased incidence of QTc outliers, either absolute or change from baseline, associated with fingolimod treatment. In MS studies, there was no clinically relevant prolongation of QT interval, but patients at risk for QT prolongation were not included in clinical studies.
Immune System
Effects on Immune Cell Numbers in the Blood
In a study in which 12 subjects received GILENYA 0.5 mg daily, the lymphocyte count decreased to approximately 60% of baseline within 4-6 hours after the first dose. With continued daily dosing, the lymphocyte count continued to decrease over a 2-week period, reaching a nadir count of approximately 500 cells/μL or approximately 30% of baseline. In a placebo-controlled study in 1272 MS patients (of whom 425 received fingolimod 0.5 mg daily and 418 received placebo), 18% (N=78) of patients on fingolimod 0.5 mg reached a nadir of <200 cells/μL on at least 1 occasion. No patient on placebo reached a nadir of <200 cells/μL. Low lymphocyte counts are maintained with chronic daily dosing of GILENYA 0.5 mg daily.
Chronic fingolimod dosing leads to a mild decrease in the neutrophil count to approximately 80% of baseline. Monocytes are unaffected by fingolimod.
Peripheral lymphocyte count increases are evident within days of stopping fingolimod treatment and typically normal counts are reached within 1 to 2 months.
Effect on Antibody Response
GILENYA reduces the immune response to vaccination, as evaluated in 2 studies.
In the first study, the immunogenicity of keyhole limpet hemocyanin (KLH) and pneumococcal polysaccharide vaccine (PPV-23) immunization were assessed by IgM and IgG titers in a steady-state, randomized, placebo-controlled study in healthy volunteers. Compared to placebo, antigen-specific IgM titers were decreased by 91% and 25% in response to KLH and PPV-23, respectively, in subjects on GILENYA 0.5 mg. Similarly, IgG titers were decreased by 45% and 50%, in response to KLH and PPV-23, respectively, in subjects on GILENYA 0.5 mg daily compared to placebo. The responder rate for GILENYA 0.5 mg as measured by the number of subjects with a >4-fold increase in KLH IgG was comparable to placebo and 25% lower for PPV-23 IgG, while the number of subjects with a >4 fold increase in KLH and PPV-23 IgM was 75% and 40% lower, respectively, compared to placebo. The capacity to mount a skin delayed-type hypersensitivity reaction to Candida and tetanus toxoid was decreased by approximately 30% in subjects on GILENYA 0.5 mg daily, compared to placebo. Immunologic responses were further decreased with fingolimod 1.25 mg (a dose higher than recommended in MS) [see Warnings and Precautions (5.2)].
In the second study, the immunogenicity of Northern hemisphere seasonal influenza and tetanus toxoid vaccination was assessed in a 12-week steady-state, randomized, placebo-controlled study of GILENYA 0.5 mg in multiple sclerosis patients (n=136). The responder rate 3 weeks after vaccination, defined as seroconversion or a ≥4-fold increase in antibody directed against at least 1 of the 3 influenza strains, was 54% for GILENYA 0.5 mg and 85% in the placebo group. The responder rate 3 weeks after vaccination, defined as seroconversion or a ≥4-fold increase in antibody directed against tetanus toxoid was 40% for GILENYA 0.5 mg and 61% in the placebo group.
Pulmonary Function
Single fingolimod doses ≥5 mg (10-fold the recommended dose) are associated with a dose-dependent increase in airway resistance. In a 14-day study of 0.5, 1.25, or 5 mg/day, fingolimod was not associated with impaired oxygenation or oxygen desaturation with exercise or an increase in airway responsiveness to methacholine. Subjects on fingolimod treatment had a normal bronchodilator response to inhaled beta-agonists.
In a 14-day placebo-controlled study of patients with moderate asthma, no effect was seen for GILENYA 0.5 mg (recommended dose in MS). A 10% reduction in mean FEV1 at 6 hours after dosing was observed in patients receiving fingolimod 1.25 mg (a dose higher than recommended for use in MS) on Day 10 of treatment. Fingolimod 1.25 mg was associated with a 5-fold increase in the use of rescue short acting beta-agonists.
12.3 Pharmacokinetics
Absorption
The Tmax of fingolimod is 12-16 hours. The apparent absolute oral bioavailability is 93%.
Food intake does not alter Cmax or exposure (AUC) of fingolimod or fingolimod-phosphate. Therefore GILENYA may be taken without regard to meals.
Steady-state blood concentrations are reached within 1 to 2 months following once-daily administration and steady-state levels are approximately 10-fold greater than with the initial dose.
Distribution
Fingolimod highly (86%) distributes in red blood cells. Fingolimod-phosphate has a smaller uptake in blood cells of <17%. Fingolimod and fingolimod-phosphate are >99.7% protein bound. Fingolimod and fingolimod-phosphate protein binding is not altered by renal or hepatic impairment.
Fingolimod is extensively distributed to body tissues with a volume of distribution of about 1200±260 L.
Metabolism
The biotransformation of fingolimod in humans occurs by 3 main pathways: by reversible stereoselective phosphorylation to the pharmacologically active (S)-enantiomer of fingolimod-phosphate, by oxidative biotransformation mainly via the cytochrome P450 4F2 isoenzyme and subsequent fatty acid-like degradation to inactive metabolites, and by formation of pharmacologically inactive non-polar ceramide analogs of fingolimod.
Fingolimod is primarily metabolized via human CYP4F2 with a minor contribution of CYP2D6, 2E1, 3A4, and 4F12. Inhibitors or inducers of these isozymes might alter the exposure of fingolimod or fingolimod-phosphate. The involvement of multiple CYP isoenzymes in the oxidation of fingolimod suggests that the metabolism of fingolimod will not be subject to substantial inhibition in the presence of an inhibitor of a single specific CYP isozyme.
Following single oral administration of [14C] fingolimod, the major fingolimod-related components in blood, as judged from their contribution to the AUC up to 816 hours post-dose of total radiolabeled components, are fingolimod itself (23.3%), fingolimod-phosphate (10.3%), and inactive metabolites [M3 carboxylic acid metabolite (8.3%), M29 ceramide metabolite (8.9%), and M30 ceramide metabolite (7.3%)].
Elimination
Fingolimod blood clearance is 6.3±2.3 L/h, and the average apparent terminal half-life (t1/2) is 6 to 9 days. Blood levels of fingolimod-phosphate decline in parallel with those of fingolimod in the terminal phase, yielding similar half-lives for both.
After oral administration, about 81% of the dose is slowly excreted in the urine as inactive metabolites. Fingolimod and fingolimod-phosphate are not excreted intact in urine but are the major components in the feces with amounts of each representing less than 2.5% of the dose.
Special Populations
Renal Impairment
In patients with severe renal impairment, fingolimod Cmax and AUC are increased by 32% and 43%, respectively, and fingolimod-phosphate Cmax and AUC are increased by 25% and 14%, respectively, with no change in apparent elimination half-life. Based on these findings, the GILENYA 0.5 mg dose is appropriate for use in patients with renal impairment. The systemic exposure of 2 metabolites (M2 and M3) is increased by 3- and 13-fold, respectively. The toxicity of these metabolites has not been fully characterized.
A study in patients with mild or moderate renal impairment has not been conducted.
Hepatic Impairment
In subjects with mild, moderate, or severe hepatic impairment, no change in fingolimod Cmax was observed, but fingolimod AUC was increased respectively by 12%, 44%, and 103%. In patients with severe hepatic impairment, fingolimod-phosphate Cmax was decreased by 22% and AUC was not substantially changed. The pharmacokinetics of fingolimod-phosphate was not evaluated in patients with mild or moderate hepatic impairment. The apparent elimination half-life of fingolimod is unchanged in subjects with mild hepatic impairment, but is prolonged by about 50% in patients with moderate or severe hepatic impairment.
Patients with severe hepatic impairment should be closely monitored, as the risk of adverse reactions is greater [see Warnings and Precautions (5.6)].
No dose adjustment is needed in patients with mild or moderate hepatic impairment.
Race
The effects of race on fingolimod and fingolimod-phosphate pharmacokinetics cannot be adequately assessed due to a low number of non-white patients in the clinical program.
Gender
Gender has no clinically significant influence on fingolimod and fingolimod-phosphate pharmacokinetics.
Geriatric Patients
The mechanism for elimination and results from population pharmacokinetics suggest that dose adjustment would not be necessary in elderly patients. However, clinical experience in patients aged above 65 years is limited.
Pharmacokinetic Interactions
Ketoconazole
The coadministration of ketoconazole (a potent inhibitor of CYP3A and CYP4F) 200 mg twice daily at steady-state and a single dose of fingolimod 5 mg led to a 70% increase in AUC of fingolimod and fingolimod-phosphate. Patients who use GILENYA and systemic ketoconazole concomitantly should be closely monitored, as the risk of adverse reactions is greater [see Drug Interactions (7)].
Carbamazepine
The coadministration of carbamazepine (a potent CYP450 enzyme inducer) 600 mg twice daily at steady-state and a single dose of fingolimod 2 mg decreased blood concentrations (AUC) of fingolimod and fingolimod-phosphate by approximately 40%. The clinical impact of this decrease is unknown.
Other strong CYP450 enzyme inducers, e.g., rifampicin, phenytoin, phenobarbital, and St. John’s wort, may also reduce AUC of fingolimod and fingolimod-phosphate. The clinical impact of this potential decrease is unknown.
Potential of Fingolimod and Fingolimod-phosphate to Inhibit the Metabolism of Comedications
In vitro inhibition studies in pooled human liver microsomes and specific metabolic probe substrates demonstrate that fingolimod has little or no capacity to inhibit the activity of the following CYP450 enzymes: CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4/5, or CYP4A9/11, and similarly fingolimod-phosphate has little or no capacity to inhibit the activity of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4 at concentrations up to 3 orders of magnitude of therapeutic concentrations. Therefore, fingolimod and fingolimod-phosphate are unlikely to reduce the clearance of drugs that are mainly cleared through metabolism by the major cytochrome P450 isoenzymes described above.
Potential of Fingolimod and Fingolimod-phosphate to Induce its Own and/or the Metabolism of Comedications
Fingolimod was examined for its potential to induce human CYP3A4, CYP1A2, CYP4F2, and MDR1 (P-glycoprotein) mRNA and CYP3A, CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP4F2 activity in primary human hepatocytes. Fingolimod did not induce mRNA or activity of the different CYP450 enzymes and MDR1 with respect to the vehicle control; therefore, no clinically relevant induction of the tested CYP450 enzymes or MDR1 by fingolimod are expected at therapeutic concentrations. Fingolimod-phosphate was also examined for its potential to induce mRNA and/or activity of human CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP3A, CYP4F2, CYP4F3B, and CYP4F12. Fingolimod-phosphate is not expected to have clinically significant induction effects on these enzymes at therapeutic dose of fingolimod.
Transporters
Fingolimod as well as fingolimod-phosphate are not expected to inhibit the uptake of comedications and/or biologics transported by OATP1B1, OATP1B3, or NTCP. Similarly, they are not expected to inhibit the efflux of comedications and/or biologics transported by the breast cancer resistant protein (MXR), the bile salt export pump (BSEP), the multidrug resistance-associated protein 2 (MRP2), and MDR1-mediated transport at therapeutic concentrations.
Oral Contraceptives
The coadministration of fingolimod 0.5 mg daily with oral contraceptives (ethinylestradiol and levonorgestrel) did not elicit any clinically significant change in oral contraceptives exposure. Fingolimod and fingolimod-phosphate exposure were consistent with those from previous studies. No interaction studies have been performed with oral contraceptives containing other progestagens; however, an effect of fingolimod on their exposure is not expected.
Cyclosporine
The pharmacokinetics of single-dose fingolimod was not altered during coadministration with cyclosporine at steady-state, nor was cyclosporine steady-state pharmacokinetics altered by fingolimod. These data indicate that GILENYA is unlikely to reduce the clearance of drugs mainly cleared by CYP3A4 and show that the potent inhibition of transporters MDR1, MRP2, and OATP-C does not influence fingolimod disposition.
Isoproterenol, Atropine, Atenolol, and Diltiazem
Single-dose fingolimod and fingolimod-phosphate exposure was not altered by coadministered isoproterenol or atropine. Likewise, the single-dose pharmacokinetics of fingolimod and fingolimod-phosphate and the steady-state pharmacokinetics of both atenolol and diltiazem were unchanged during the coadministration of the latter 2 drugs individually with fingolimod.
Population Pharmacokinetics Analysis
A population pharmacokinetics evaluation performed in MS patients did not provide evidence for a significant effect of fluoxetine and paroxetine (strong CYP2D6 inhibitors) on fingolimod or fingolimod-phosphate predose concentrations. In addition, the following commonly coprescribed substances had no clinically relevant effect (<20%) on fingolimod or fingolimod-phosphate predose concentrations: baclofen, gabapentin, oxybutynin, amantadine, modafinil, amitriptyline, pregabalin, and corticosteroids.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Oral carcinogenicity studies of fingolimod were conducted in mice and rats. In mice, fingolimod was administered at oral doses of 0, 0.025, 0.25, and 2.5 mg/kg/day for up to 2 years. The incidence of malignant lymphoma was increased in males and females at the mid and high dose. The lowest dose tested (0.025 mg/kg/day) is less than the recommended human dose (RHD) of 0.5 mg/day on a body surface area (mg/m2) basis. In rats, fingolimod was administered at oral doses of 0, 0.05, 0.15, 0.5, and 2.5 mg/kg/day. No increase in tumors was observed. The highest dose tested (2.5 mg/kg/day) is approximately 50 times the RHD on a mg/m2 basis.
Fingolimod was negative in a battery of in vitro (Ames, mouse lymphoma thymidine kinase, chromosomal aberration in mammalian cells) and in vivo (micronucleus in mouse and rat) assays.
When fingolimod was administered orally (0, 1, 3, and 10 mg/kg/day) to male and female rats prior to and during mating, and continuing to Day 7 of gestation in females, no effect on fertility was observed up to the highest dose tested (10 mg/kg), which is approximately 200 times the RHD on a mg/m2 basis.
13.2 Animal Toxicology and/or Pharmacology
Lung toxicity was observed in 2 different strains of rats and in dogs and monkeys. The primary findings included increase in lung weight, associated with smooth muscle hypertrophy, hyperdistension of the alveoli, and/or increased collagen. Insufficient or lack of pulmonary collapse at necropsy, generally correlated with microscopic changes, was observed in all species. In rats and monkeys, lung toxicity was observed at all oral doses tested in chronic studies. The lowest doses tested in rats (0.05 mg/kg/day in the 2-year carcinogenicity study) and monkeys (0.5 mg/kg/day in the 39-week toxicity study) are similar to and approximately 20 times the RHD on a mg/m2 basis, respectively.
In the 52-week oral study in monkeys, respiratory distress associated with ketamine administration was observed at doses of 3 and 10 mg/kg/day; the most affected animal became hypoxic and required oxygenation. As ketamine is not generally associated with respiratory depression, this effect was attributed to fingolimod. In a subsequent study in rats, ketamine was shown to potentiate the bronchoconstrictive effects of fingolimod. The relevance of these findings to humans is unknown.
14 CLINICAL STUDIES
The efficacy of GILENYA was demonstrated in 2 studies that evaluated once-daily doses of GILENYA 0.5 mg and 1.25 mg in patients with relapsing-remitting MS (RRMS). Both studies included patients who had experienced at least 2 clinical relapses during the 2 years prior to randomization or at least 1 clinical relapse during the 1 year prior to randomization, and had an Expanded Disability Status Scale (EDSS) score from 0 to 5.5. Study 1 was a 2-year randomized, double-blind, placebo-controlled study in patients with RRMS who had not received any interferon-beta or glatiramer acetate for at least the previous 3 months and had not received any natalizumab for at least the previous 6 months. Neurological evaluations were performed at screening, every 3 months and at time of suspected relapse. MRI evaluations were performed at screening, Month 6, Month 12, and Month 24. The primary endpoint was the annualized relapse rate.
Median age was 37 years, median disease duration was 6.7 years and median EDSS score at baseline was 2.0. Patients were randomized to receive GILENYA 0.5 mg (N=425), 1.25 mg (N=429), or placebo (N=418) for up to 24 months. Median time on study drug was 717 days on 0.5 mg, 715 days on 1.25 mg and 719 days on placebo.
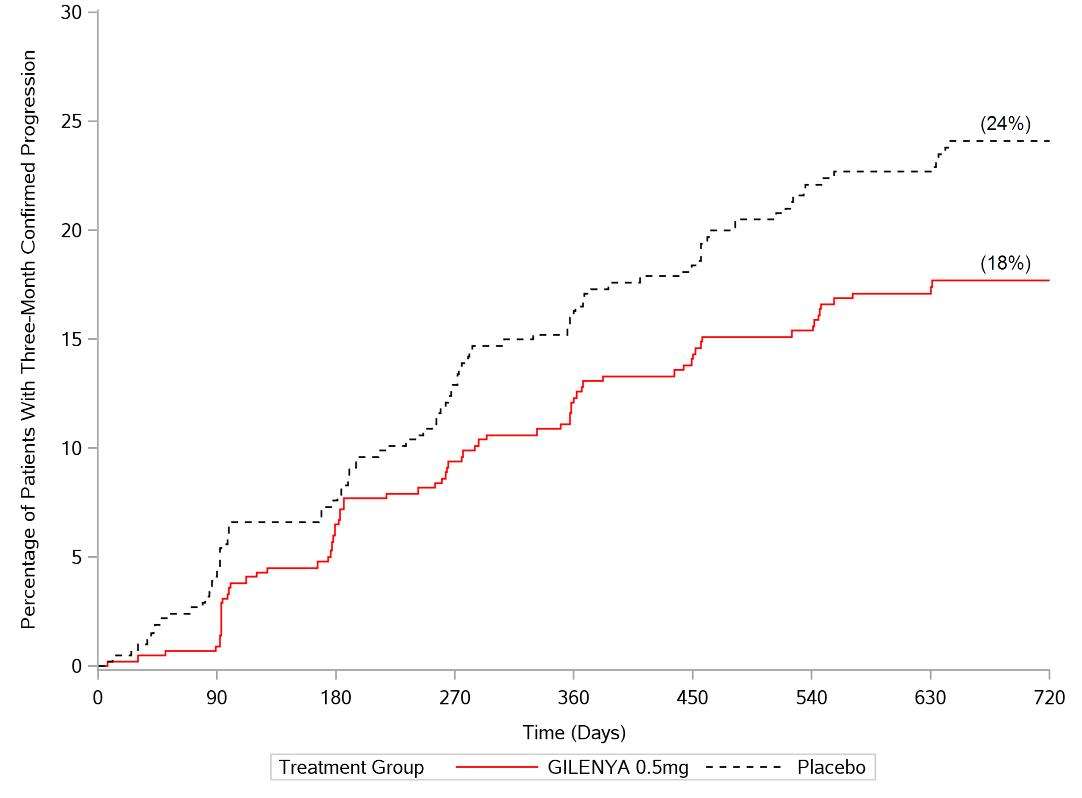
The annualized relapse rate was significantly lower in patients treated with GILENYA than in patients who received placebo. The secondary endpoint was the time to 3-month confirmed disability progression as measured by at least a 1-point increase from baseline in EDSS (0.5 point increase for patients with baseline EDSS of 5.5) sustained for 3 months. Time to onset of 3-month confirmed disability progression was significantly delayed with GILENYA treatment compared to placebo. The 1.25 mg dose resulted in no additional benefit over the GILENYA 0.5 mg dose. The results for this study are shown in Table 2 and Figure 1.
| All analyses of clinical endpoints were intent-to–treat. MRI analysis used evaluable dataset. ‡ Hazard ratio is an estimate of the relative risk of having the event of disability progression on GILENYA as compared to placebo. |
|||
| GILENYA 0.5 mg N=425 | Placebo N=418 | p-value | |
| Clinical Endpoints | |||
| Annualized relapse rate (primary endpoint) | 0.18 | 0.40 | <0.001 |
| Percentage of patients without relapse | 70% | 46% | <0.001 |
| Hazard ratio‡ of disability progression (95% CI) |
0.70 (0.52, 0.96) |
0.02 | |
| MRI Endpoint | |||
| Mean (median) number of new or newly enlarging T2 lesions over 24 months | 2.5 (0) | 9.8 (5.0) | <0.001 |
| Mean (median) number of T1 Gd-enhancing lesions at Month 24 | 0.2 (0) | 1.1 (0) | <0.001 |
Study 2 was a 1-year randomized, double-blind, double-dummy, active-controlled study in patients with RRMS who had not received any natalizumab in the previous 6 months. Prior therapy with interferon-beta or glatiramer acetate up to the time of randomization was permitted.
Neurological evaluations were performed at screening, every 3 months, and at the time of suspected relapses. MRI evaluations were performed at screening and at month 12. The primary endpoint was the annualized relapse rate.
Median age was 36 years, median disease duration was 5.9 years, and median EDSS score at baseline was 2.0. Patients were randomized to receive GILENYA 0.5 mg (N=431), 1.25 mg (N=426), or interferon beta-1a, 30 micrograms via the intramuscular route (IM) once weekly (N=435) for up to 12 months. Median time on study drug was 365 days on GILENYA 0.5 mg, 354 days on 1.25 mg, and 361 days on interferon beta-1a IM.
The annualized relapse rate was significantly lower in patients treated with GILENYA 0.5 mg than in patients who received interferon beta-1a IM. The key secondary endpoints were number of new and newly enlarging T2 lesions and time to onset of 3-month confirmed disability progression as measured by at least a 1-point increase from baseline in EDSS (0.5 point increase for those with baseline EDSS of 5.5) sustained for 3 months. The number of new and newly enlarging T2 lesions was significantly lower in patients treated with GILENYA than in patients who received interferon beta-1a IM. There was no significant difference in the time to 3-month confirmed disability progression between GILENYA and interferon beta-1a-treated patients at 1 year. The 1.25 mg dose resulted in no additional benefit over the GILENYA 0.5 mg dose. The results for this study are shown in Table 3.
| All analyses of clinical endpoints were intent-to–treat. MRI analysis used evaluable dataset. ‡ Hazard ratio is an estimate of the relative risk of having the event of disability progression on GILENYA as compared to control. |
|||
| GILENYA 0.5 mg N=429 | Interferon beta-1a IM 30 μg N=431 | p-value | |
| Clinical Endpoints | |||
| Annualized relapse rate (primary endpoint) | 0.16 | 0.33 | <0.001 |
| Percentage of patients without relapse | 83% | 70% | <0.001 |
| Hazard ratio‡ of disability progression (95% CI) |
0.71 (0.42, 1.21) |
0.21 | |
| MRI Endpoint | |||
| Mean (median) number of new or newly enlarging T2 lesions over 12 months | 1.6 (0) | 2.6 (1.0) | 0.002 |
| Mean (median) number of T1 Gd-enhancing lesions at Month 12 | 0.2 (0) | 0.5 (0) | <0.001 |
Pooled results of study 1 and study 2 showed a consistent and statistically significant reduction of annualized relapse rate compared to comparator in subgroups defined by gender, age, prior MS therapy, and disease activity.
16 HOW SUPPLIED/STORAGE AND HANDLING
0.5 mg GILENYA capsules are hard gelatin capsules with a white opaque body and bright yellow cap imprinted with “FTY 0.5 mg” on the cap and 2 radial bands imprinted on the capsule body with yellow ink.
GILENYA capsules are supplied in blister packs.
Carton of 28 capsules containing 2 folded blister cards of 14 capsules per blister card NDC 0078-0607-51
Carton of 7 capsules containing 1 blister card of 7 capsules per blister card NDC 0078-0607-89
GILENYA capsules should be stored at 25ºC (77ºF); excursions permitted to 15ºC–30ºC (59ºF–86ºF). Protect from moisture.
17 PATIENT COUNSELING INFORMATION
See Medication Guide.
A Medication Guide is required for distribution with GILENYA. Encourage patients to read the GILENYA Medication Guide. The complete text of the Medication Guide is reprinted at the end of this document.
Benefits and Risks
Summarize for patients the benefits and potential risks of treatment with GILENYA. Tell patients to take GILENYA once daily as prescribed. Tell patients not to discontinue GILENYA without first discussing this with the prescribing physician. Advise patients to contact their physician if they accidently take more Gilenya than prescribed.
Cardiac Effects
Advise patients that initiation of GILENYA treatment results in a transient decrease in heart rate. Inform patients that they will need to be observed in the doctor's office or other facility for at least 6 hours after the first dose. Advise patients that if GILENYA is discontinued for more than 14 days, effects similar to those observed on treatment initiation may be seen and observation for at least 6 hours will be needed on treatment reinitiation, and that the same precautions will be taken if treatment is interrupted for more than 1 day within the first 2 weeks of treatment, or for more than 7 days during week 3 and 4 of treatment.
Risk of Infections
Inform patients that they may be more likely to get infections when taking GILENYA, and that they should contact their physician if they develop symptoms of infection. Advise patients that the use of some vaccines should be avoided during treatment with GILENYA and for 2 months after discontinuation. Advise patients who have not had chickenpox or vaccination to consider VZV vaccination prior to commencing treatment with GILENYA. Inform patients that prior or concomitant use of drugs that suppress the immune system may increase the risk of infection.
Macular Edema
Advise patients that GILENYA may cause macular edema, and that they should contact their physician if they experience any changes in their vision. Inform patients with diabetes mellitus or a history of uveitis that their risk of macular edema is increased.
Respiratory Effects
Advise patients that they should contact their physician if they experience new onset or worsening of dyspnea.
Hepatic Effects
Inform patients that GILENYA may increase liver enzymes. Advise patients that they should contact their physician if they have any unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine.
Fetal Risk
Inform patients that, based on animal studies, GILENYA may cause fetal harm. Discuss with women of childbearing age whether they are pregnant, might be pregnant or are trying to become pregnant. Advise women of childbearing age of the need for effective contraception during GILENYA treatment and for 2 months after stopping GILENYA. Advise the patient that if she should nevertheless become pregnant, she should immediately inform her physician.
Persistence of GILENYA Effects After Drug Discontinuation
Advise patients that GILENYA remains in the blood and continues to have effects, including decreased blood lymphocyte counts, for up to 2 months following the last dose.
T2014-53
April 2014
MEDICATION GUIDE
GILENYA® (je-LEN-yah)
(fingolimod)
capsules
Read this Medication Guide before you start using Gilenya and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your health problem or treatment.
What is the most important information I should know about GILENYA?
GILENYA may cause serious side effects, including:
1. Slow heart rate (bradycardia or bradyarrhythmia) when you start taking GILENYA. GILENYA can cause your heart rate to slow down, especially after you take your first dose. You will have a test to check the electrical activity of your heart (ECG) before you take your first dose of GILENYA.
You will be observed by a healthcare professional for at least 6 hours after you take your first dose of GILENYA.
After you take your first dose of GILENYA:
- Your pulse and blood pressure should be checked every hour.
- You should be observed by a healthcare professional to see if you have any serious side effects. If your heart rate slows down too much, you may have symptoms such as:
- dizziness
- tiredness
- feeling like your heart is beating slowly or skipping beats
- dizziness
- If you have any of the symptoms of slow heart rate, they will usually happen during the first 6 hours after your first dose of GILENYA. Symptoms can happen up to 24 hours after you take your first GILENYA dose.
- 6 hours after you take your first dose of GILENYA you will have another ECG. If your ECG shows any heart problems or if your heart rate is still too low or continues to decrease, you will continue to be observed.
- If you have any serious side effects after your first dose of GILENYA, especially those that require treatment with other medicines, you will stay in the medical facility to be observed overnight. You will also be observed for any serious side effects for at least 6 hours after you take your second dose of GILENYA the next day.
- If you have certain types of heart problems, or if you are taking certain types of medicines that can affect your heart, you will be observed overnight after you take your first dose of GILENYA.
Your slow heart rate will usually return to normal within 1 month after you start taking GILENYA. Call your doctor or go to the nearest hospital emergency room right away if you have any symptoms of a slow heart rate.
If you miss 1 or more doses of GILENYA you may need to be observed by a healthcare professional when you take your next dose. Call your doctor if you miss a dose of GILENYA. See “How should I take GILENYA?”
2. Infections. GILENYA can increase your risk of serious infections and decrease the way vaccines work in your body to prevent certain diseases, especially the chicken pox vaccine. GILENYA lowers the number of white blood cells (lymphocytes) in your blood. This will usually go back to normal within 2 months of stopping treatment. Your doctor may do a blood test before you start taking GILENYA. Call your doctor right away if you have any of these symptoms of an infection:
- fever
- tiredness
- body aches
- chills
- nausea
- vomiting
3. A problem with your vision called macular edema. Macular edema can cause some of the same vision symptoms as an MS attack (optic neuritis). You may not notice any symptoms with macular edema. If macular edema happens, it usually starts in the first 3 to 4 months after you start taking GILENYA. Your doctor should test your vision before you start taking GILENYA and 3 to 4 months after you start taking GILENYA, or any time you notice vision changes during treatment with GILENYA. Your risk of macular edema may be higher if you have diabetes or have had an inflammation of your eye called uveitis.
Call your doctor right away if you have any of the following:
- blurriness or shadows in the center of your vision
- a blind spot in the center of your vision
- sensitivity to light
- unusually colored (tinted) vision
What is GILENYA?
GILENYA is a prescription medicine used to treat relapsing forms of multiple sclerosis (MS) in adults. GILENYA can decrease the number of MS flare-ups (relapses). GILENYA does not cure MS, but it can help slow down the physical problems that MS causes.
It is not known if GILENYA is safe and effective in children under 18 years of age.
Who should not take GILENYA?
Do not take GILENYA if you:
- have had a heart attack, unstable angina, stroke or warning stroke or certain types of heart failure in the last 6 months
- have certain types of irregular or abnormal heartbeat (arrhythmia), including patients in whom a heart finding called prolonged QT is seen on ECG before starting GILENYA
- are taking certain medicines that change your heart rhythm
If any of the above situations apply to you, tell your doctor.
What should I tell my doctor before taking GILENYA?
Before you take GILENYA, tell your doctor about all your medical conditions, including if you had or now have:
- an irregular or abnormal heartbeat (arrhythmia)
- a history of stroke or warning stroke
- heart problems, including heart attack or angina
- a history of repeated fainting (syncope)
- a fever or infection, or you are unable to fight infections due to a disease or taking medicines that lower your immune system. Tell your doctor if you have had chicken pox or have received the vaccine for chicken pox. Your doctor may do a blood test for chicken pox virus. You may need to get the vaccine for chicken pox and then wait 1 month before you start taking GILENYA.
- eye problems, especially an inflammation of the eye called uveitis.
- diabetes
- breathing problems, including during your sleep
- liver problems
- high blood pressure
- Are pregnant or plan to become pregnant. GILENYA may harm your unborn baby. Talk to your doctor if you are pregnant or are planning to become pregnant.
- Tell your doctor right away if you become pregnant while taking GILENYA or if you become pregnant within 2 months after you stop taking GILENYA.
- If you are a female who can become pregnant, you should use effective birth control during your treatment with GILENYA and for at least 2 months after you stop taking GILENYA.
Pregnancy Registry: There is a registry for women who become pregnant during treatment with GILENYA. If you become pregnant while taking GILENYA, talk to your doctor about registering with the GILENYA Pregnancy Registry. The purpose of this registry is to collect information about your health and your baby’s health.
For more information, contact the GILENYA Pregnancy Registry by calling Outcome at 1-877-598-7237, by sending an email to gpr@outcome.com, or go to www.gilenyapregnancyregistry.com.
- Tell your doctor right away if you become pregnant while taking GILENYA or if you become pregnant within 2 months after you stop taking GILENYA.
- Are breastfeeding or plan to breastfeed. It is not known if GILENYA passes into your breast milk. You and your doctor should decide if you will take GILENYA or breastfeed. You should not do both.
Tell your doctor about all the medicines you take or have recently taken, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your doctor if you take medicines that affect your immune system, or have taken them in the past.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist when you get a new medicine.
Using GILENYA and other medicines together may affect each other causing serious side effects.
Especially tell your doctor if you take vaccines. Tell your doctor if you have been vaccinated within 1 month before you start taking GILENYA. You should not get certain vaccines while you take GILENYA and for at least 2 months after you stop taking GILENYA. If you take certain vaccines, you may get the infection the vaccine should have prevented. Vaccines may not work as well when given during GILENYA treatment.
How should I take GILENYA?
- You will be observed by a healthcare professional for at least 6 hours after your first dose of GILENYA. See “What is the most important information I should know about GILENYA?”
- Take GILENYA exactly as your doctor tells you to take it.
- Take GILENYA 1 time each day.
- If you take too much GILENYA, call your doctor or go to the nearest hospital emergency room right away.
- Take GILENYA with or without food.
- Do not stop taking GILENYA without talking with your doctor first.
- Call your doctor right away if you miss a dose of GILENYA. You may need to be observed by a healthcare professional for at least 6 hours when you take your next dose. If you need to be observed by a healthcare professional when you take your next dose of GILENYA you will have:
- an ECG before you take your dose
- hourly pulse and blood pressure measurements after you take the dose
- an ECG 6 hours after your dose
- If you have certain types of heart problems, or if you are taking certain types of medicines that can affect your heart, you will be observed overnight by a healthcare professional in a medical facility after you take your dose of GILENYA.
- If you have serious side effects after taking a dose of GILENYA, especially those that require treatment with other medicines, you will stay in the medical facility to be observed overnight. If you were observed overnight, you will also be observed for any serious side effects for at least 6 hours after you take your second dose of GILENYA. See “What is the most important information I should know about GILENYA?”
What are possible side effects of GILENYA?
GILENYA can cause serious side effects.
See "What is the most important information I should know about GILENYA?"
Serious side effects include:
-
swelling and narrowing of the blood vessels in your brain that may lead to a stroke or bleeding. This problem usually gets better when you stop taking GILENYA. Call your doctor right away if you have any of the following symptoms of a stroke or bleeding in your brain, including:
- sudden headache
- confusion
- seizures
- loss of vision
- weakness
- breathing problems. Some people who take GILENYA have shortness of breath. Call your doctor right away if you have trouble breathing.
-
liver problems. GILENYA may cause liver problems. Your doctor should do blood tests to check your liver before you start taking GILENYA. Call your doctor right away if you have any of the following symptoms of liver problems:
- nausea
- vomiting
- stomach pain
- loss of appetite
- tiredness
- your skin or the whites of your eyes turn yellow
- dark urine
The most common side effects of GILENYA include:
- headache
- flu
- diarrhea
- back pain
- abnormal liver tests
- cough
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of GILENYA. For more information, ask your doctor or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store GILENYA?
- Store GILENYA in the original blister pack in a dry place.
- Store GILENYA at room temperature between 59°F to 86°F (15°C to 30°C).
- Keep GILENYA and all medicines out of the reach of children.
General information about GILENYA
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use GILENYA for a condition for which it was not prescribed. Do not give GILENYA to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about GILENYA. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about GILENYA that is written for healthcare professionals.
For more information, go to www.pharma.US.Novartis.com or call 1-888-669-6682.
What are the ingredients in GILENYA?
Active ingredient: fingolimod
Inactive ingredients: gelatin, magnesium stearate, mannitol, titanium dioxide, yellow iron oxide.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
GILENYA is a registered trademark of Novartis AG.
Manufactured by:
Novartis Pharma Stein AG
Stein, Switzerland
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
© Novartis
T2014-54
Revised: April 2014
Package Label – 0.5 mg
Rx Only NDC 0078-0607-51
GILENYA™
(fingolimod)
Capsules
28 Capsules
0.5 mg
Equivalent to 0.56 mg fingolimod hydrochloride
This package contains a four-week supply of capsules.
Dispense with enclosed Medication Guide.
GilenyaFingolimod hcl CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||