Glipizide
FULL PRESCRIBING INFORMATION: CONTENTS*
- GLIPIZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS & USAGE
- GLIPIZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LABORATORY TESTS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- GLIPIZIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
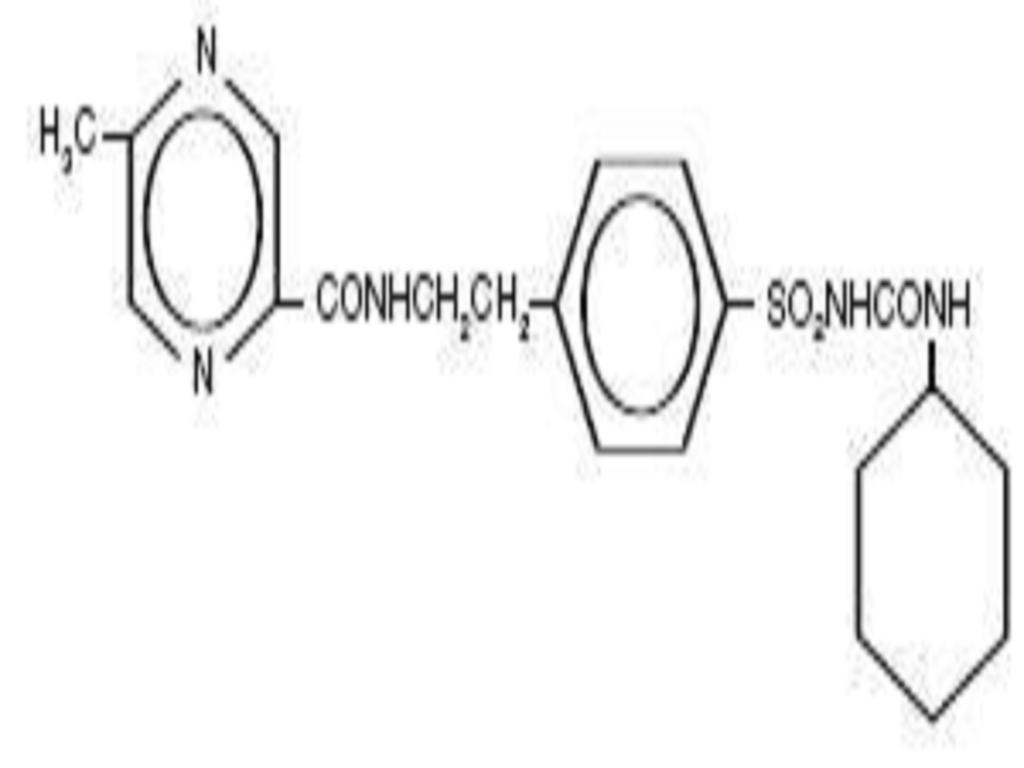
GLIPIZIDE DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanism of Action:Pharmacokinetics
Other Effects:
PHARMACOKINETICS
INDICATIONS & USAGE
GLIPIZIDE CONTRAINDICATIONS
WARNINGS
SPECIAL WARNING ON INCREASED RISK OF CARDIOVASCULAR MORTALITY:The administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetics Program (UGDP), a long-term prospective clinical trial designed to evaluate the effectiveness of glucose-lowering drugs in preventing or delaying vascular complications in patients with non-insulin-dependent diabetes. The study involved 823 patients who were randomly assigned to one of four treatment groups. (Diabetes, 19, supp. 2:747-830, 1970).
UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of tolbutamide (1.5 grams per day) had a rate of cardiovascular mortality approximately 2times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results, the findings of the UGDP study provide an adequate basis for this warning. The patient should be informed of the potential risks and advantages of glipizide and of alternative modes of therapy.
Although only one drug in the sulfonylurea class (tolbutamide) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other oral hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure.
PRECAUTIONS
GeneralRenal and Hepatic Disease:
Hypoglycemia:
Loss of Control of Blood Glucose
LABORATORY TESTS
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic Effects:Nonteratogenic Effects:
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
GLIPIZIDE ADVERSE REACTIONS
Hypoglycemia:PRECAUTIONSOVERDOSAGE
Gastrointestinal:
Dermatologic:
Hematologic:
Metabolic:
Endocrine Reactions:
Miscellaneous:
Laboratory Tests:
OVERDOSAGE
DOSAGE & ADMINISTRATION
Initial Dose:
Titration:
Maintenance:
PRECAUTIONS
Patients Receiving Insulin:
Patients Receiving Other Oral Hypoglycemic Agents:
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

GlipizideGlipizide TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!