Glipizide
Revised: January 2014
FULL PRESCRIBING INFORMATION: CONTENTS*
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Glipizide is an oral blood-glucose-lowering drug of the sulfonylurea class.
The Chemical Abstracts name of glipizide is 1-Cyclohexyl-3-[[p-[2-(5-methylpyrazinecarboxamido)ethyl]phenyl]sulfonyl]urea. The molecular formula is C21H27N5O4S; the molecular weight is 445.55; the structural formula is shown below:
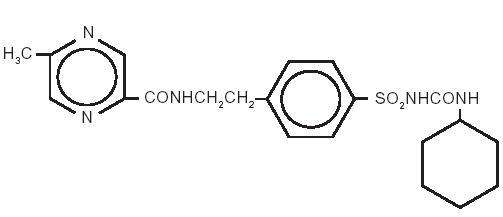
Glipizide is a whitish, odorless powder with a pKa of 5.9. It is insoluble in water and alcohols, but soluble in 0.1 N NaOH; it is freely soluble in dimethylformamide.
Glipizide extended-release tablets are formulated as a polymer matrix based once-a-day controlled release tablet for oral use and is designed to deliver 2.5 mg, 5 mg or 10 mg of glipizide. Each tablet contains the following inactive ingredients: acetyltributyl citrate, edible black ink, hydroxyethyl cellulose, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, methacrylic acid copolymer type A and polyethylene glycol.
The 2.5 mg and 5 mg tablets also contain FD&C Yellow #6.
Glipizide appears to lower blood glucose acutely by stimulating the release of insulin from the pancreas, an effect dependent upon functioning beta cells in the pancreatic islets. Extrapancreatic effects also may play a part in the mechanism of action of oral sulfonylurea hypoglycemic drugs. Two extrapancreatic effects shown to be important in the action of glipizide are an increase in insulin sensitivity and a decrease in hepatic glucose production. However, the mechanism by which glipizide lowers blood glucose during long-term administration has not been clearly established. Stimulation of insulin secretion by glipizide in response to a meal is of major importance. The insulinotropic response to a meal is enhanced with glipizide administration in diabetic patients. The postprandial insulin and C-peptide responses continue to be enhanced after at least 6 months of treatment. In 2 randomized, double-blind, dose-response studies comprising a total of 347 patients, there was no significant increase in fasting insulin in all glipizide-treated patients combined compared to placebo, although minor elevations were observed at some doses. There was no increase in fasting insulin over the long term.
Some patients fail to respond initially, or gradually lose their responsiveness to sulfonylurea drugs, including glipizide. Alternatively, glipizide may be effective in some patients who have not responded or have ceased to respond to other sulfonylureas.
The effectiveness of glipizide extended-release tablets in type 2 diabetes at doses from 5-60 mg once daily has been evaluated in 4 therapeutic clinical trials each with long-term open extensions involving a total of 598 patients. Once daily administration of 5 mg, 10 mg and 20 mg produced statistically significant reductions from placebo in hemoglobin A1C, fasting plasma glucose and postprandial glucose in patients with mild to severe type 2 diabetes. In a pooled analysis of the patients treated with 5 mg and 20 mg, the relationship between dose and glipizide extended-release tablet's effect of reducing hemoglobin A1C was not established. However, in the case of fasting plasma glucose, patients treated with 20 mg had a statistically significant reduction of fasting plasma glucose compared to the 5 mg-treated group.
The reductions in hemoglobin A1C and fasting plasma glucose were similar in younger and older patients. Efficacy of glipizide extended-release tablets was not affected by gender, race or weight (as assessed by body mass index). In long term extension trials, efficacy of glipizide extended-release tablets was maintained in 81% of patients for up to 12 months.
In an open, two-way crossover study 132 patients were randomly assigned to either glipizide extended-release tablets or glipizide tablets for 8 weeks and then crossed over to the other drug for an additional 8 weeks. Glipizide extended-release tablets administration resulted in significantly lower fasting plasma glucose levels and equivalent hemoglobin A1C levels, as compared to glipizide tablets.
In 12 week, well-controlled studies, there was a maximal average net reduction in hemoglobin A1C of 1.7% in absolute units between placebo-treated and Glipizide extended-release tablet-treated patients.
It has been shown that glipizide extended-release tablet therapy is effective in controlling blood glucose without deleterious changes in the plasma lipoprotein profiles of patients treated for type 2 diabetes.
In a placebo-controlled, crossover study in normal volunteers, glipizide had no antidiuretic activity, and, in fact, led to a slight increase in free water clearance.
Glipizide is rapidly and completely absorbed following oral administration in an immediate release dosage form. The absolute bioavailability of glipizide was 100% after single oral doses in patients with type 2 diabetes. Beginning 2 to 3 hours after administration of glipizide extended-release tablets, plasma drug concentrations gradually rise reaching maximum concentrations within 6 to 12 hours after dosing. With subsequent once daily dosing of glipizide extended-release tablets, effective plasma glipizide concentrations are maintained throughout the 24 hour dosing interval with less peak to trough fluctuation than that observed with twice daily dosing of immediate release glipizide. The mean relative bioavailability of glipizide in 21 males with type 2 diabetes after administration of 20 mg glipizide extended-release tablets, compared to immediate release glipizide (10 mg given twice daily), was 90% at steady-state. Steady-state plasma concentrations were achieved by at least the fifth day of dosing with glipizide extended-release tablets in 21 males with type 2 diabetes and patients younger than 65 years. Approximately 1 to 2 days longer were required to reach steady-state in 24 elderly (≥65 years) males and females with type 2 diabetes. No accumulation of drug was observed in patients with type 2 diabetes during chronic dosing with glipizide extended-release tablets. Administration of glipizide extended-release tablets with food has no effect on the 2 to 3 hour lag time in drug absorption. In a single dose, food effect study in 21 healthy male subjects, the administration of glipizide extended-release tablets immediately before a high fat breakfast resulted in a 40% increase in the glipizide mean Cmax value, which was significant, but the effect on the AUC was not significant. There was no change in glucose response between the fed and fasting state. Markedly reduced GI retention times of the glipizide extended-release tablets over prolonged periods (e.g., short bowel syndrome) may influence the pharmacokinetic profile of the drug and potentially result in lower plasma concentrations. In a multiple dose study in 26 males with type 2 diabetes, the pharmacokinetics of glipizide were linear over the dose range of 5 to 60 mg of glipizide extended-release tablets in that the plasma drug concentrations increased proportionately with dose. In a single dose study in 24 healthy subjects, four 5 mg, two 10 mg, and one 20 mg glipizide extended-release tablets were bioequivalent. In a separate single dose study in 36 healthy subjects, four 2.5 mg glipizide extended-release tablets were bioequivalent to one 10-mg glipizide extended-release tablet.
Glipizide is eliminated primarily by hepatic biotransformation: less than 10% of a dose is excreted as unchanged drug in urine and feces; approximately 90% of a dose is excreted as biotransformation products in urine (80%) and feces (10%). The major metabolites of glipizide are products of aromatic hydroxylation and have no hypoglycemic activity. A minor metabolite which accounts for less than 2% of a dose, an acetylamino-ethyl benzene derivative, is reported to have 1/10 to 1/3 as much hypoglycemic activity as the parent compound. The mean total body clearance of glipizide was approximately 3 liters per hour after single intravenous doses in patients with type 2 diabetes. The mean apparent volume of distribution was approximately 10 liters. Glipizide is 98 to 99% bound to serum proteins, primarily to albumin. The mean terminal elimination half-life of glipizide ranged from 2 to 5 hours after single or multiple doses in patients with type 2 diabetes. There were no significant differences in the pharmacokinetics of glipizide after single dose administration to older diabetic subjects compared to younger healthy subjects. There is only limited information regarding the effects of renal impairment on the disposition of glipizide, and no information regarding the effects of hepatic disease. However, since glipizide is highly protein bound and hepatic biotransformation is the predominant route of elimination, the pharmacokinetics and/or pharmacodynamics of glipizide may be altered in patients with renal or hepatic impairment.
In mice no glipizide or metabolites were detectable autoradiographically in the brain or spinal cord of males or females, nor in the fetuses of pregnant females. In another study, however, very small amounts of radioactivity were detected in the fetuses of rats given labeled drug.
Glipizide extended-release tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Glipizide extended-release tablets are contraindicated in patients with:
-
Known hypersensitivity to glipizide or any excipients in the tablets.
-
Type 1 diabetes mellitus, diabetic ketoacidosis, with or without coma. This condition should be treated with insulin.
SPECIAL WARNING ON INCREASED RISK OF CARDIOVASCULAR MORTALITY:
The administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetes Program (UGDP), a long-term prospective clinical trial designed to evaluate the effectiveness of glucose-lowering drugs in preventing or delaying vascular complications in patients with type 2 diabetes. The study involved 823 patients who were randomly assigned to one of four treatment groups (
Diabetes
, 19, SUPP. 2: 747-830, 1970).
UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of tolbutamide (1.5 grams per day) had a rate of cardiovascular mortality approximately 2 1/2 times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results, the findings of the UGDP study provide an adequate basis for this warning. The patient should be informed of the potential risks and advantages of glipizide and of alternative modes of therapy.
Although only one drug in the sulfonylurea class (tolbutamide) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other oral hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure.
As with any other non-deformable material, caution should be used when administering glipizide extended-release tablets in patients with pre-existing severe gastrointestinal narrowing (pathologic or iatrogenic). There have been rare reports of obstructive symptoms in patients with known strictures in association with the ingestion of another drug in this non-deformable sustained release formulation.
Macrovascular Outcomes: There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with glipizide extended-release tablets or any other anti-diabetic drug.
Renal and Hepatic Disease: The pharmacokinetics and/or pharmacodynamics of glipizide may be affected in patients with impaired renal or hepatic function. If hypoglycemia should occur in such patients, it may be prolonged and appropriate management should be instituted.
GI Disease: Markedly reduced GI retention times of the glipizide extended-release tablets may influence the pharmacokinetic profile and hence the clinical efficacy of the drug.
Hypoglycemia: All sulfonylurea drugs are capable of producing severe hypoglycemia. Proper patient selection, dosage, and instructions are important to avoid hypoglycemic episodes. Renal or hepatic insufficiency may affect the disposition of glipizide and the latter may also diminish gluconeogenic capacity, both of which increase the risk of serious hypoglycemic reactions. Elderly, debilitated or malnourished patients, and those with adrenal or pituitary insufficiency are particularly susceptible to the hypoglycemic action of glucose-lowering drugs. Hypoglycemia may be difficult to recognize in the elderly, and in people who are taking beta-adrenergic blocking drugs. Hypoglycemia is more likely to occur when caloric intake is deficient, after severe or prolonged exercise, when alcohol is ingested, or when more than one glucose-lowering drug is used. Therapy with a combination of glucose-lowering agents may increase the potential for hypoglycemia.
Loss of Control of Blood Glucose: When a patient stabilized on any diabetic regimen is exposed to stress such as fever, trauma, infection, or surgery, a loss of control may occur. At such times, it may be necessary to discontinue glipizide and administer insulin.
The effectiveness of any oral hypoglycemic drug, including glipizide, in lowering blood glucose to a desired level decreases in many patients over a period of time, which may be due to progression of the severity of the diabetes or to diminished responsiveness to the drug. This phenomenon is known as secondary failure, to distinguish it from primary failure in which the drug is ineffective in an individual patient when first given. Adequate adjustment of dose and adherence to diet should be assessed before classifying a patient as a secondary failure.
Hemolytic Anemia: Treatment of patients with glucose 6-phosphate dehydrogenase (G6PD) deficiency with sulfonylurea agents can lead to hemolytic anemia. Because glipizide belongs to the class of sulfonylurea agents, caution should be used in patients with G6PD deficiency and a non-sulfonylurea alternative should be considered. In postmarketing reports, hemolytic anemia has also been reported in patients who did not have known G6PD deficiency.
Blood and urine glucose should be monitored periodically. Measurement of hemoglobin A1C may be useful.
Patients should be informed that glipizide extended-release tablets should be swallowed whole. Patients should not chew, divide or crush tablets.
Patients should be informed of the potential risks and advantages of glipizide extended-release tablets and of alternative modes of therapy. They should also be informed about the importance of adhering to dietary instructions, of a regular exercise program, and of regular testing of urine and/or blood glucose.
The risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development should be explained to patients and responsible family members. Primary and secondary failure also should be explained.
Physician Counseling Information for Patients:
In initiating treatment for type 2 diabetes, diet should be emphasized as the primary form of treatment. Caloric restriction and weight loss are essential in the obese diabetic patient. Proper dietary management alone may be effective in controlling the blood glucose and symptoms of hyperglycemia. The importance of regular physical activity should also be stressed, and cardiovascular risk factors should be identified and corrective measures taken where possible. Use of glipizide extended-release tablets or other antidiabetic medications must be viewed by both the physician and patient as a treatment in addition to diet and not as a substitution or as a convenient mechanism for avoiding dietary restraint. Furthermore, loss of blood glucose control on diet alone may be transient, thus requiring only short-term administration of glipizide extended-release tablets or other antidiabetic medications. Maintenance or discontinuation of glipizide extended-release tablets or other antidiabetic medications should be based on clinical judgment using regular clinical and laboratory evaluations.
The hypoglycemic action of sulfonylureas may be potentiated by certain drugs including nonsteroidal anti-inflammatory agents and other drugs that are highly protein bound, salicylates, sulfonamides, chloramphenicol, probenecid, coumarins, monoamine oxidase inhibitors, and beta-adrenergic blocking agents. When such drugs are administered to a patient receiving glipizide, the patient should be observed closely for hypoglycemia. When such drugs are withdrawn from a patient receiving glipizide, the patient should be observed closely for loss of control. In vitro binding studies with human serum proteins indicate that glipizide binds differently than tolbutamide and does not interact with salicylate or dicumarol. However, caution must be exercised in extrapolating these findings to the clinical situation and in the use of glipizide with these drugs.
Certain drugs tend to produce hyperglycemia and may lead to loss of control. These drugs include the thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid. When such drugs are administered to a patient receiving glipizide, the patient should be closely observed for loss of control. When such drugs are withdrawn from a patient receiving glipizide, the patient should be observed closely for hypoglycemia.
A potential interaction between oral miconazole and oral hypoglycemic agents leading to severe hypoglycemia has been reported. Whether this interaction also occurs with the intravenous, topical, or vaginal preparations of miconazole is not known. The effect of concomitant administration of fluconazole and glipizide has been demonstrated in a placebo-controlled crossover study in normal volunteers. All subjects received glipizide alone and following treatment with 100 mg of fluconazole as a single daily oral dose for 7 days. The mean percentage increase in the glipizide AUC after fluconazole administration was 56.9% (range: 35 to 81%).
In studies assessing the effect of colesevelam on the pharmacokinetics of glipizide ER in healthy volunteers, reductions in glipizide AUC0-∞ and Cmax of 12% and 13%, respectively were observed when colesevelam was coadministered with glipizide ER. When glipizide ER was administered 4 hours prior to colesevelam, there was no significant change in glipizide AUC0-∞ or Cmax, -4% and 0%, respectively. Therefore, glipizide ER should be administered at least 4 hours prior to colesevelam to ensure that colesevelam does not reduce the absorption of glipizide.
A twenty month study in rats and an eighteen month study in mice at doses up to 75 times the maximum human dose revealed no evidence of drug-related carcinogenicity. Bacterial and in vivo mutagenicity tests were uniformly negative. Studies in rats of both sexes at doses up to 75 times the human dose showed no effects on fertility.
Pregnancy Category C:
Glipizide was found to be mildly fetotoxic in rat reproductive studies at all dose levels (5 to 50 mg/kg). This fetotoxicity has been similarly noted with other sulfonylureas, such as tolbutamide and tolazamide. The effect is perinatal and believed to be directly related to the pharmacologic (hypoglycemic) action of glipizide. In studies in rats and rabbits no teratogenic effects were found. There are no adequate and well controlled studies in pregnant women. Glipizide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Because recent information suggests that abnormal blood-glucose levels during pregnancy are associated with a higher incidence of congenital abnormalities, many experts recommend that insulin be used during pregnancy to maintain blood-glucose levels as close to normal as possible.
Prolonged severe hypoglycemia (4 to 10 days) has been reported in neonates born to mothers who were receiving a sulfonylurea drug at the time of delivery. This has been reported more frequently with the use of agents with prolonged half-lives. If glipizide is used during pregnancy, it should be discontinued at least one month before the expected delivery date.
Although it is not known whether glipizide is excreted in human milk, some sulfonylurea drugs are known to be excreted in human milk. Because the potential for hypoglycemia in nursing infants may exist, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. If the drug is discontinued and if diet alone is inadequate for controlling blood glucose, insulin therapy should be considered.
Safety and effectiveness in children have not been established.
Of the total number of patients in clinical studies of glipizide extended-release tablets, 33 percent were 65 and over. Approximately 1 to 2 days longer were required to reach steady-state in the elderly. (See CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION .)There were no overall differences in effectiveness or safety between younger and older patients, but greater sensitivity of some individuals cannot be ruled out. As such, it should be noted that elderly, debilitated or malnourished patients, and those with adrenal or pituitary insufficiency, are particularly susceptible to the hypoglycemic action of glucose-lowering drugs. Hypoglycemia may be difficult to recognize in the elderly. In addition, in elderly, debilitated or malnourished patients, and patients with impaired renal or hepatic function, the initial and maintenance dosing should be conservative to avoid hypoglycemic reactions.
In U.S. controlled studies the frequency of serious adverse experiences reported was very low and causal relationship has not been established.
The 580 patients from 31 to 87 years of age who received glipizide extended-release tablets in doses from 5 mg to 60 mg in both controlled and open trials were included in the evaluation of adverse experiences. All adverse experiences reported were tabulated independently of their possible causal relation to medication.
Hypoglycemia: See PRECAUTIONS and OVERDOSAGE sections.
Only 3.4% of patients receiving glipizide extended-release tablets had hypoglycemia documented by a blood-glucose measurement <60 mg/dL and/or symptoms believed to be associated with hypoglycemia. In a comparative efficacy study of glipizide extended-release tablets and glipizide tablets, hypoglycemia occurred rarely with an incidence of less than 1% with both drugs.
In double-blind, placebo-controlled studies the adverse experiences reported with an incidence of 3% or more in glipizide extended-release tablet-treated patients include:
| Glipizide ER (%) | Placebo (%) | |
| Adverse Effect | (N= 278) | (N=69) |
| Asthenia | 10.1 | 13.0 |
| Headache | 8.6 | 8.7 |
| Dizziness | 6.8 | 5.8 |
| Nervousness | 3.6 | 2.9 |
| Tremor | 3.6 | 0.0 |
| Diarrhea | 5.4 | 0.0 |
| Flatulence | 3.2 | 1.4 |
The following adverse experiences occurred with an incidence of less than 3% in glipizide extended-release tablet-treated patients:
Body as a whole–pain
Nervous system–insomnia, paresthesia, anxiety, depression and hypesthesia
Gastrointestinal–nausea, dyspepsia, constipation and vomiting
Metabolic–hypoglycemia
Musculoskeletal–arthralgia, leg cramps and myalgia
Cardiovascular–syncope
Skin–sweating and pruritus
Respiratory–rhinitis
Special senses–blurred vision
Urogenital–polyuria
Other adverse experiences occurred with an incidence of less than 1% in glipizide extended-release tablet-treated patients:
Body as a whole–chills
Nervous system–hypertonia, confusion, vertigo, somnolence, gait abnormality and decreased libido
Gastrointestinal–anorexia and trace blood in stool
Metabolic–thirst and edema
Cardiovascular–arrhythmia, migraine, flushing and hypertension
Skin–rash and urticaria
Respiratory–pharyngitis and dyspnea
Special senses–pain in the eye, conjunctivitis and retinal hemorrhage
Urogenital–dysuria
Although these adverse experiences occurred in patients treated with glipizide extended-release tablets, a causal relationship to the medication has not been established in all cases.
There have been rare reports of gastrointestinal irritation and gastrointestinal bleeding with use of another drug in this non-deformable sustained release formulation, although causal relationship to the drug is uncertain.
Postmarketing Experience
The following adverse events have been reported in postmarketing surveillance:
Gastrointestinal: abdominal pain
Hepatobiliary: Cholestatic and hepatocellular forms of liver injury accompanied by jaundice have been reported rarely in association with glipizide; glipizide extended-release tablets should be discontinued if this occurs.
The following are adverse experiences reported with immediate release glipizide and other sulfonylureas, but have not been observed with glipizide extended-release tablets:
Hematologic: Leukopenia, agranulocytosis, thrombocytopenia, hemolytic anemia (see PRECAUTIONS ), aplastic anemia and pancytopenia have been reported with sulfonylureas.
Metabolic: Hepatic porphyria and disulfiram-like reactions have been reported with sulfonylureas. In the mouse, glipizide pretreatment did not cause an accumulation of acetaldehyde after ethanol administration. Clinical experience to date has shown that glipizide has an extremely low incidence of disulfiram-like alcohol reactions.
Endocrine Reactions: Cases of hyponatremia and the syndrome of inappropriate antidiuretic hormone (SIADH) secretion have been reported with glipizide and other sulfonylureas.
Laboratory Tests: The pattern of laboratory test abnormalities observed with glipizide was similar to that for other sulfonylureas. Occasional mild to moderate elevations of SGOT, LDH, alkaline phosphatase, BUN and creatinine were noted. One case of jaundice was reported. The relationship of these abnormalities to glipizide is uncertain, and they have rarely been associated with clinical symptoms.
There is no well-documented experience with glipizide extended-release tablets overdosage in humans. There have been no known suicide attempts associated with purposeful overdosing with glipizide extended-release tablets. In nonclinical studies the acute oral toxicity of glipizide was extremely low in all species tested (LD50 greater than 4 g/kg). Overdosage of sulfonylureas including glipizide can produce hypoglycemia. Mild hypoglycemic symptoms without loss of consciousness or neurologic findings should be treated aggressively with oral glucose and adjustments in drug dosage and/or meal patterns. Close monitoring should continue until the physician is assured that the patient is out of danger. Severe hypoglycemic reactions with coma, seizure, or other neurological impairment occur infrequently, but constitute medical emergencies requiring immediate hospitalization. If hypoglycemic coma is diagnosed or suspected, the patient should be given rapid intravenous injection of concentrated (50%) glucose solution. This should be followed by a continuous infusion of a more dilute (10%) glucose solution at a rate that will maintain the blood glucose at a level above 100 mg/dL. Patients should be closely monitored for a minimum of 24 to 48 hours since hypoglycemia may recur after apparent clinical recovery. Clearance of glipizide from plasma may be prolonged in persons with liver disease. Because of the extensive protein binding of glipizide, dialysis is unlikely to be of benefit.
There is no fixed dosage regimen for the management of diabetes mellitus with glipizide extended-release tablets or any other hypoglycemic agent. Glycemic control should be monitored with hemoglobin A1C and/or blood-glucose levels to determine the minimum effective dose for the patient; to detect primary failure, i.e., inadequate lowering of blood glucose at the maximum recommended dose of medication; and to detect secondary failure, i.e., loss of an adequate blood-glucose-lowering response after an initial period of effectiveness. Home blood-glucose monitoring may also provide useful information to the patient and physician. Short-term administration of glipizide extended-release tablets may be sufficient during periods of transient loss of control in patients usually controlled on diet.
In general, glipizide extended-release tablets should be given with breakfast.
Recommended Dosing: The usual starting dose of glipizide extended-release tablets as initial therapy is 5 mg per day, given with breakfast. Those patients who may be more sensitive to hypoglycemic drugs may be started at a lower dose.
Dosage adjustment should be based on laboratory measures of glycemic control. While fasting blood-glucose levels generally reach steady-state following initiation or change in glipizide extended-release tablet dosage, a single fasting glucose determination may not accurately reflect the response to therapy. In most cases, hemoglobin A1C level measured at three month intervals is the preferred means of monitoring response to therapy.
Hemoglobin A1C should be measured as glipizide extended-release tablet therapy is initiated and repeated approximately three months later. If the result of this test suggests that glycemic control over the preceding three months was inadequate, the glipizide extended-release tablet dose may be increased. Subsequent dosage adjustments should be made on the basis of hemoglobin A1C levels measured at three month intervals. If no improvement is seen after three months of therapy with a higher dose, the previous dose should be resumed. Decisions which utilize fasting blood glucose to adjust glipizide extended-release tablet therapy should be based on at least two or more similar, consecutive values obtained seven days or more after the previous dose adjustment.
Most patients will be controlled with 5 mg to 10 mg taken once daily. However, some patients may require up to the maximum recommended daily dose of 20 mg. While the glycemic control of selected patients may improve with doses which exceed 10 mg, clinical studies conducted to date have not demonstrated an additional group average reduction of hemoglobin A1C beyond what was achieved with the 10 mg dose.
Based on the results of a randomized crossover study, patients receiving immediate release glipizide may be switched safely to glipizide extended-release tablets once-a-day at the nearest equivalent total daily dose. Patients receiving immediate release glipizide also may be titrated to the appropriate dose of glipizide extended-release tablets starting with 5 mg once daily. The decision to switch to the nearest equivalent dose or to titrate should be based on clinical judgment.
In elderly patients, debilitated or malnourished patients, and patients with impaired renal or hepatic function, the initial and maintenance dosing should be conservative to avoid hypoglycemic reactions (see PRECAUTIONS section).
Combination Use: When adding other blood-glucose-lowering agents to glipizide extended-release tablets for combination therapy, the agent should be initiated at the lowest recommended dose, and patients should be observed carefully for hypoglycemia. Refer to the product information supplied with the oral agent for additional information.
When adding glipizide extended-release tablets to other blood-glucose-lowering agents, glipizide extended-release tablets can be initiated at 5 mg. Those patients who may be more sensitive to hypoglycemic drugs may be started at a lower dose. Titration should be based on clinical judgment.
When colesevelam is coadministered with glipizide ER, maximum plasma concentration and total exposure to glipizide is reduced. Therefore, glipizide ER should be administered at least 4 hours prior to colesevelam.
Patients Receiving Insulin: As with other sulfonylurea-class hypoglycemics, many patients with stable type 2 diabetes receiving insulin may be transferred safely to treatment with glipizide extended-release tablets. When transferring patients from insulin to glipizide extended-release tablets, the following general guidelines should be considered:
For patients whose daily insulin requirement is 20 units or less, insulin may be discontinued and glipizide extended-release tablet therapy may begin at usual dosages. Several days should elapse between titration steps.
For patients whose daily insulin requirement is greater than 20 units, the insulin dose should be reduced by 50% and glipizide extended-release tablet therapy may begin at usual dosages. Subsequent reductions in insulin dosage should depend on individual patient response. Several days should elapse between titration steps.
During the insulin withdrawal period, the patient should test urine samples for sugar and ketone bodies at least three times daily. Patients should be instructed to contact the prescriber immediately if these tests are abnormal. In some cases, especially when the patient has been receiving greater than 40 units of insulin daily, it may be advisable to consider hospitalization during the transition period.
Patients Receiving Other Oral Hypoglycemic Agents: As with other sulfonylurea-class hypoglycemics, no transition period is necessary when transferring patients to glipizide extended-release tablets. Patients should be observed carefully (1 to 2 weeks) for hypoglycemia when being transferred from longer half-life sulfonylureas (e.g., chlorpropamide) to glipizide extended-release tablets due to potential overlapping of drug effect.
Glipizide Extended-Release Tablets are supplied as 2.5 mg, 5 mg, and 10 mg round, film-coated tablets and are printed with black ink as follows:
2.5 mg tablets are light orange and printed with WPI and 900, and are supplied in bottles of 30, 100 and 1000.
5 mg tablets are orange and printed with WPI and 844, and are supplied in bottles of 100 and 1000 and blister packages of 30.
10 mg tablets are white to off-white and printed with WPI and 845, and are supplied in bottles of 100 and 1000 and blister packages of 30.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Protect from moisture and humidity.
Mfd. for:
Watson Laboratories, Inc.
Corona, CA 92880 USA
Mfd. by:
Patheon Pharmaceuticals Inc.
Cincinnati, OH 45237 USA
Revised: January 2014
Glipizide ER (Extended-Release Tablets)
Read this information carefully before you start taking this medicine. Read the information you get with your medicine each time you refill your prescription. There may be new information. This information does not take the place of your healthcare provider’s advice. Ask your healthcare provider or pharmacist if you do not understand some of this information or if you want to know more about this medicine.
What is Glipizide Extended-release tablets?
Glipizide Extended-release tablets is a medicine you take by mouth. It is used to treat type 2 diabetes (also called non-insulin-dependent diabetes mellitus). Your healthcare provider has prescribed Glipizide Extended-release tablets because your current treatment, including diet and exercise, has not been able to bring your blood sugar level under good control.
Your body makes insulin to keep the amount of sugar (glucose) in your blood at the right level. With type 2 diabetes:
- your body may not be making enough insulin
- your body may not be using the insulin that you have already made
- the level of sugar in your blood is too high
If your blood sugar level is not under control, it can lead to serious medical problems, such as kidney damage, nerve damage, blindness, problems with circulation (blood movement in your body), loss of feet, legs, or other limbs, high blood pressure, heart attack, or stroke.
Glipizide Extended-release tablets work mainly by:
- helping the body release more of its own insulin
- helping the body respond better to its own insulin
- lowering the amount of sugar (glucose) made by the body
Even after you start taking Glipizide Extended-release tablets, you must continue to follow your program of diet and exercise.
Who Should Not Use Glipizide Extended-release tablets?
Do not use Glipizide Extended-release tablets if you:
- have a condition called diabetic ketoacidosis
- have ever had an allergic reaction to glipizide or any of the other ingredients in Glipizide Extended-release tablets. Ask your healthcare provider or pharmacist for a list of these ingredients.
Only your healthcare provider can decide if Glipizide Extended-release tablets are right for you. Before you start Glipizide Extended-release tablets, tell the healthcare provider if you:
- are taking or using any prescription medicines or nonprescription medicines, including natural or herbal remedies. Other medications can increase your chance of getting low blood sugar or high blood sugar. Be sure to tell your healthcare provider if you take the medicines miconazole or fluconazole, used to fight fungus infections.
- have ever had a condition called diabetic ketoacidosis
- have kidney or liver problems
- have had blockage or narrowing of your intestines due to illness or past surgery
- have chronic (continuing) diarrhea
- are pregnant or might be pregnant. Your healthcare provider may switch you to insulin injections some time during your pregnancy. You should not take Glipizide Extended-release tablets during the last month of pregnancy.
- are breast-feeding. Glipizide may pass to the baby through your milk and cause harm.
- have glucose-6-phosphate dehydrogenase (G6PD) deficiency. This condition usually runs in families. People with G6PD deficiency who take Glipizide Extended-release tablets may develop hemolytic anemia (fast breakdown of red blood cells).
How Should I Take Glipizide Extended-release tablets?
Glipizide Extended-release tablets come in three different strengths (2.5 mg, 5 mg and 10 mg). Your healthcare provider will prescribe the dose that is right for you.
- Take Glipizide Extended-release tablets once a day with breakfast. The tablet is designed to release the medicine slowly over 24 hours. This is why you have to take it only once a day.
- Swallow the tablet whole. Never chew, crush, or cut the tablet in half. This would damage the tablet and release too much medicine into your body at one time.
It is important to take Glipizide Extended-release tablets every day to help keep your blood sugar level under good control. Your healthcare provider may adjust your dose depending on your blood glucose test results. If your blood sugar level is not under control, call your healthcare provider. Do not change your dose without your healthcare provider's approval.
In case of overdose, call the poison control center or your healthcare provider right away, or have someone drive you to the nearest emergency room.
You must continue your diet and exercise program while taking Glipizide Extended-release tablets. You must also have your blood and urine tested regularly to be sure Glipizide Extended-release tablets are working.
Glipizide Extended-release tablets may not work for everyone. If it does work, you may find that Glipizide Extended-release tablets are not working as well for you after you have used it for a while. Tell your healthcare provider if Glipizide Extended-release tablets are not working well.
What Should I Avoid While Taking Glipizide Extended-release tablets?
Some medicines can affect how well Glipizide Extended-release tablets work or may affect your blood sugar level. Check with your healthcare provider or pharmacist before you start or stop taking prescription or over-the-counter medicines, including natural/herbal remedies, while on Glipizide Extended-release tablets.
What are the Possible Side Effects of Glipizide Extended-release tablets?
Low blood sugar. Glipizide Extended-release tablets may lower your blood sugar to low levels that are dangerous (hypoglycemia). This can happen if you do not follow your diet, exercise too much, drink alcohol, are under stress, or get sick. This could also happen if your dose of Glipizide Extended-release tablets is higher than you need. Your healthcare provider may need to adjust it. Do not adjust the dose on your own.
Be sure you know how to recognize your body’s signs that your blood sugar is too low. These signs include:
|
• a cold clammy feeling |
• hunger |
|
• unusual sweating |
• fast heartbeat |
|
• dizziness |
• headache |
|
• weakness |
• blurred vision |
|
• trembling |
• slurred speech |
|
• shakiness |
• tingling in the lips or hands |
If you notice any of these signs, eat or drink something with sugar in it right away, such as a regular (not diet) soft drink, orange juice, honey, sugar candy. You can also keep glucose tablets on hand that are available from your pharmacy. If you do not feel better shortly or your blood sugar level does not go up, call your healthcare provider. If you cannot reach your healthcare provider in an emergency, call 911 or have someone drive you to the nearest emergency room.
Other side effects. Glipizide Extended-release tablets may cause other side effects in some people. However, the incidence of serious side effects with Glipizide Extended-release tablets are very low. Other than the signs of low blood sugar listed above, possible side effects include:
- feeling jittery
- diarrhea
- gas
Glipizide Extended-release tablets may cause other less common side effects besides those listed here. For a list of all side effects that have been reported, ask your healthcare provider or pharmacist.
While it has never been reported with Glipizide Extended-release tablets, another similar type of diabetes medicine has been linked to a higher risk of heart attacks. If you have risk factors for heart disease and take Glipizide Extended-release tablets, be sure to see your healthcare provider for regular checkups.
How To Store Glipizide Extended-release tablets
Keep Glipizide Extended-release tablets and all medicines out of the reach of children. Store Glipizide Extended-release tablets in a dry place, in its original container, and at room temperature (between 68° to 77°F or 20° to 25°C).
General Advice About Prescription Medicines
Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. If you have any concerns about Glipizide Extended-release tablets, ask your healthcare provider. Your healthcare provider or pharmacist can give you information about Glipizide Extended-release tablets that was written for healthcare professionals. Do not use Glipizide Extended-release tablets for a condition for which it was not prescribed. Do not share Glipizide Extended-release tablets with other people. For more information about Glipizide Extended-release tablets, you can visit the Watson internet site at www.watson.com.
Mfd.for: Watson Laboratories, Inc.
Corona, CA 92880 USA
Mfd.by: Patheon Pharmaceuticals Inc.
Cincinnati, OH 45237 USA
Revised: January 2014
PRINCIPAL DISPLAY PANEL
NDC 0591-0900-30
GlipiZIDE
Extended-release
Tablets
2.5 mg
Watson 30 Tablets Rx only
Each extended-release tablet contains:
Glipizide USP, 2.5 mg
Dosage: See package insert for dosage and full prescribing information.
Dispense in a tight container as defined in USP.
Store at 20º - 25º C (68º -77º F). [See USP controlled room temperature.]
Protect from moisture and humidity.
Manufactured By:
Patheon Pharmaceuticals Inc.
Cincinnati, OH 45237 USA
Distributed by Watson Pharma Inc.

PRINCIPAL DISPLAY PANEL
NDC 0591-0844-15
GlipiZIDE
Extended-Release
Tablets (anti-diabetic agent)
5 mg
Watson Rx only
1 tablet dispenser
30 tablets each
Each Extended Release tablet contains:
Glipizide USP, 5 mg
Dosage: See package insert for dosage and full prescribing information.
Dispense in a tight container as described in the USP.
Store at 20º to 25ºC (68º to 77ºF). [See USP Controlled Room Temperature.]
Mfd for:
Watson Laboratories, Inc.
Corona, CA 92880 USA
Mfd By:
Patheon Pharmaceuticals Inc.
Cincinnati, OH 43215 USA

PRINCIPAL DISPLAY PANEL
NDC 0591-0844-01
GlipiZIDE
Extended-release
Tablets
5 mg
Watson 100 Tablets Rx only
Each extended-release tablet contains:
Glipizide USP, 5 mg
Dosage: See package insert for dosage and full prescribing information.
Dispense in a tight container as defined in USP.
Store at 20º-25ºC (68º-77ºF). [See USP controlled room temperature.]
Protect from moisture and humidity.
Manufactured By:
Patheon Pharmaceuticals Inc.
Cincinnati, OH 45237 USA
Distributed by: Watson Pharma, Inc.

PRINCIPAL DISPLAY PANEL
NDC 0591-0845-15
GlipiZIDE
Extended-Release
Tablets (anti-diabetic agent)
10 mg
Watson Rx only
1 tablet dispenser
30 tablets each
Each Extended Release tablet contains:
Glipizide USP, 10 mg
Dosage: See package insert for dosage and full prescribing information.
Dispense in a tight container as described in the USP.
Store at 20º to 25ºC (68º to 77ºF). [See USP Controlled Room Temperature.]
Mfd for:
Watson Laboratories, Inc.
Corona, CA 92880 USA
Mfd By:
Patheon Pharmaceuticals Inc.
Cincinnati, OH 43215 USA
PRINCIPAL DISPLAY PANEL
NDC 0591-0845-01
GlipiZIDE
Extended-release
Tablets
10 mg
Watson 100 tablets Rx only
Each extended-release tablet contains:
Glipizide USP, 10 mg
Dosage: See package insert for dosage and full prescribing information.
Dispense in a tight container as defined in USP.
Store at 20º -25ºC (68º -77ºF). [See USP controlled room temperature.]
Protect from light and humidity.
Manufactured By:
Patheon Pharmaceuticals Inc.
Cincinnati, OH 45237 USA
Distributed by: Watson Pharma, Inc.

GlipizideGlipizide TABLET, FILM COATED, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
GlipizideGlipizide TABLET, FILM COATED, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
GlipizideGlipizide TABLET, FILM COATED, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||