GloStrips
GloStrips 1.0
FULL PRESCRIBING INFORMATION: CONTENTS*
- INDICATIONS
- DIRECTIONS FOR USE
- HOW SUPPLIED
- INSTRUCTIONS FOR OPENING STERILE GLOSTRIPS
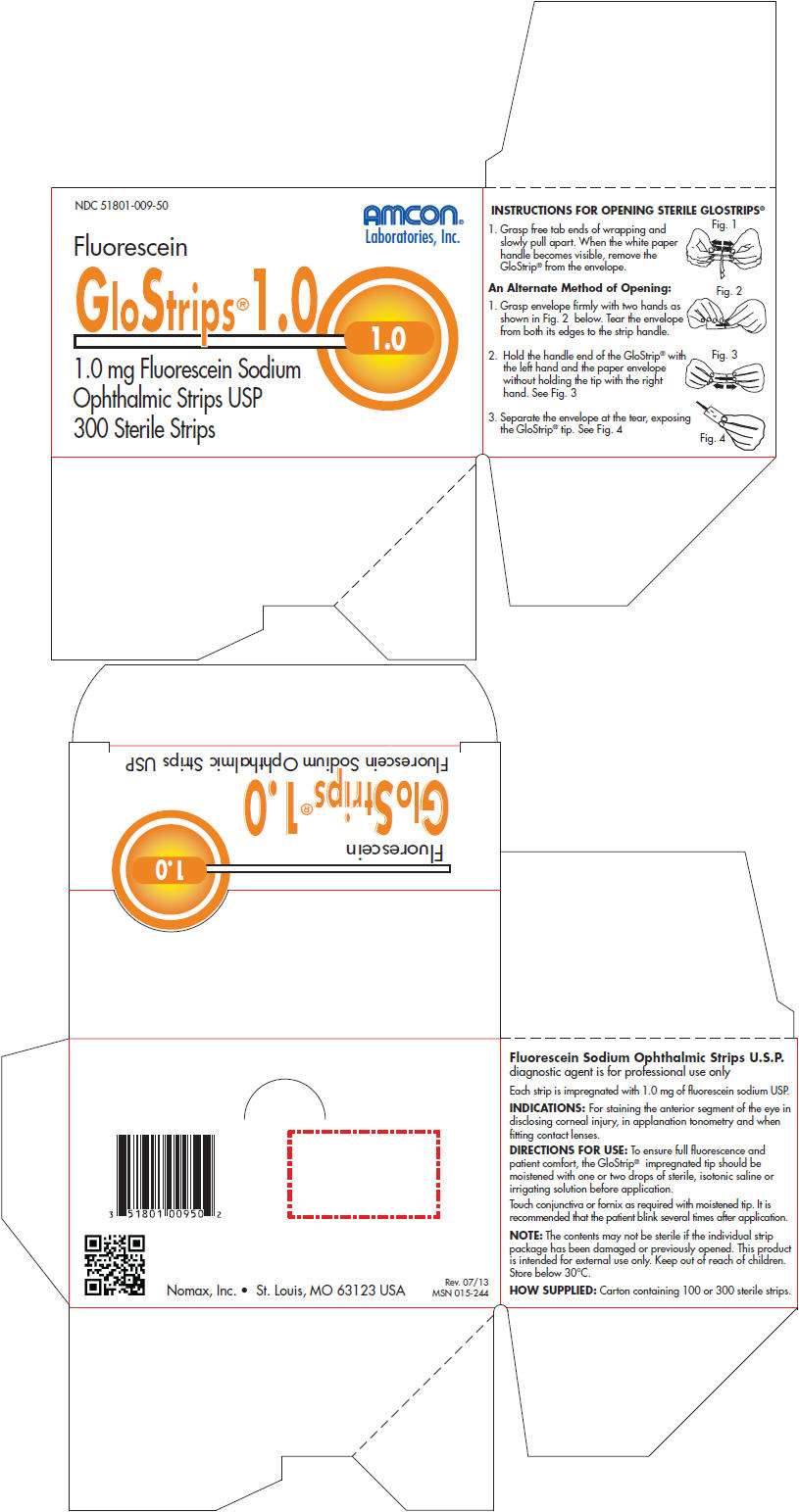
- PRINCIPAL DISPLAY PANEL - 300 Strip Carton
FULL PRESCRIBING INFORMATION
Fluorescein Sodium Ophthalmic Strips U.S.P. diagnostic agent is for professional use only
Each strip is impregnated with 1.0 mg of fluorescein sodium USP.
INDICATIONS
For staining the anterior segment of the eye in disclosing corneal injury, in applanation tonometry and when fitting contact lenses.
DIRECTIONS FOR USE
To ensure full fluorescence and patient comfort, the GloStrip® impregnated tip should be moistened with one or two drops of sterile, isotonic saline or irrigating solution before application.
Touch conjunctiva or fornix as required with moistened tip. It is recommended that the patient blink several times after application.
NOTE: The contents may not be sterile if the individual strip package has been damaged or previously opened. This product is intended for external use only. Keep out of reach of children. Store below 30°C.
HOW SUPPLIED
Carton containing 100 or 300 sterile strips.
Nomax, Inc. • St. Louis, MO 63123 USA
Rev. 07/13
MSN 015-244
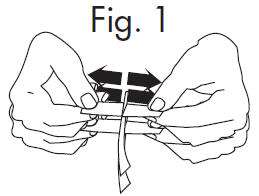
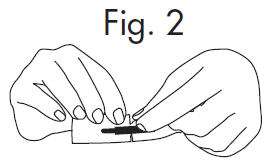
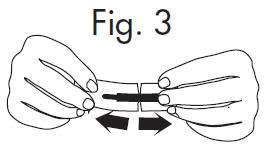
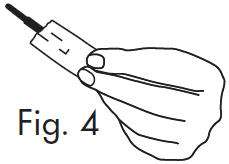
INSTRUCTIONS FOR OPENING STERILE GLOSTRIPS
|
|
An Alternate Method of Opening
|
|
|
|
|
|
PRINCIPAL DISPLAY PANEL - 300 Strip Carton
NDC 51801-009-50
AMCON®
Laboratories, Inc.
Fluorescein
GloStrips®1.0
1.0
1.0 mg Fluorescein Sodium
Ophthalmic Strips USP
300 Sterile Strips
GloStripsFluorescein Sodium STRIP
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||