Glycine
1.5% Glycine Irrigation USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- GLYCINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- GLYCINE INDICATIONS AND USAGE
- GLYCINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- GLYCINE ADVERSE REACTIONS
- GLYCINE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Directions for Use of Plastic Container
- PRINCIPAL DISPLAY PANEL - 3000 mL
FULL PRESCRIBING INFORMATION
GLYCINE DESCRIPTION
Each 100 mL contains:
Glycine USP ..................................................................................... 1.5 g
Water for Injection USP ................................................................... qs
pH: 6.1 (4.5-6.5)
Calculated Osmolarity: 200 mOsmol/liter
The formula of the active ingredient is:
| Ingredient | Molecular Formula | Molecular Weight |
|---|---|---|
| Glycine USP | NH2CH2COOH | 75.07 |
1.5% Glycine Irrigation USP is a sterile, nonpyrogenic aqueous solution suitable for urologic irrigation. This solution is slightly hypotonic.
The plastic container is made from a multilayered film specifically developed for parenteral drugs. It contains no plasticizers and exhibits virtually no leachables. The solution contact layer is a rubberized copolymer of ethylene and propylene. The container is nontoxic and biologically inert. The container-solution unit is a closed system and is not dependent upon entry of external air during administration. The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
Not made with natural rubber latex, PVC or DEHP.
CLINICAL PHARMACOLOGY
1.5% Glycine Irrigation USP is nontoxic and nonhemolytic with a refractive index close to that of water. These properties make it useful as a urologic irrigating fluid, particularly during transurethral surgical procedures where the irrigant may be absorbed through cut venous sinuses.
Systemically absorbed glycine is primarily metabolized by transamination to serine, and by deamination to ammonia which is normally removed by its conversion to urea. A portion of the absorbed glycine is excreted by the kidneys.
GLYCINE INDICATIONS AND USAGE
1.5% Glycine Irrigation USP is indicated as an irrigant during transurethral prostatic or bladder surgery.
GLYCINE CONTRAINDICATIONS
1.5% Glycine Irrigation USP is not for injection. It is contraindicated in patients with anuria.
WARNINGS
Solutions for urologic irrigation must be used with caution in patients with severe cardiopulmonary or renal dysfunction.
The amount of fluid absorbed during a transurethral resection is a function of the volume and hydrostatic pressure of the irrigant used, the number and size of venous sinuses opened, and the duration of the procedure. Since irrigating fluids used during transurethral prostatectomy have been demonstrated to enter the systemic circulation in relatively large volumes, any irrigation solution must be regarded as a systemic drug.
Absorption of large amounts of fluids containing glycine and the resultant osmotic diuresis may significantly affect cardiopulmonary and renal dynamics. Absorption of large amounts of irrigating solution can also result in pronounced hyponatremia and water intoxication.
After opening container, the contents should be used promptly to minimize the possibility of bacterial growth or pyrogen formation.
Discard unused portion of irrigating solution since it contains no preservative.
PRECAUTIONS
Cardiovascular status, especially in patients with cardiac disease, should be carefully determined before and during transurethral resection of the prostate when using 1.5% Glycine Irrigation USP as an irrigant. The fluid absorbed into the systemic circulation via severed prostatic veins may produce significant extracellular fluid expansion and lead to fulminating congestive heart failure.
Shift of sodium-free intracellular fluid into the extracellular compartment following systemic absorption of 1.5% Glycine Irrigation USP may lower serum sodium concentration and aggravate pre-existing hyponatremia.
Excessive loss of water and electrolytes may lead to serious imbalances. Sustained diuresis from transurethral irrigation with 1.5% Glycine Irrigation USP may obscure and intensify inadequate hydration or hypovolemia.
Care should be exercised if impaired liver function is known or suspected. Under such conditions, ammonia resulting from the metabolism of glycine may accumulate in the blood.
Use only if solution is clear and container and seal are intact.
GLYCINE ADVERSE REACTIONS
Adverse reactions may include fluid and electrolyte disorders such as acidosis, electrolyte loss, marked diuresis, urinary retention, edema and dehydration; cardiovascular/pulmonary disorders such as pulmonary congestion, hypotension, tachycardia, angina-like pain and thrombophlebitis; other possible reactions include visual disturbances, convulsions, nausea, vomiting, diarrhea, vertigo and urticaria.
Literature includes reports on hyponatremia, hyperammonemia, transient blindness, coma (immediate or delayed), and digitalis toxicity in digitalized patients.
If an adverse reaction occurs, discontinue the irrigant and reevaluate the clinical status of the patient.
GLYCINE DOSAGE AND ADMINISTRATION
As required for irrigation.
1.5% Glycine Irrigation USP should be administered only by the appropriate transurethraI urologic instrumentation.
It has been reported that absorption is significantly increased if the container is higher than 60 cm (24") above the patient.
This drug product should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HOW SUPPLIED
1.5% Glycine Irrigation USP is supplied sterile and nonpyrogenic in single-dose 3000 mL flexible irrigation containers packaged 4 per case.
| NDC | REF | Size |
|---|---|---|
| 0264-7390-60 | R8406 | 3000 mL |
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. It is recommended that the product be stored at room temperature (25°C); however, brief exposure up to 40°C does not adversely affect the product.
Rx only
Initiated: October 2013
Directions for Use of Plastic Container
Not for injection.
Aseptic technique is required.
- Inspect irrigation bag: overwrap and primary bag.
- Do not use if overwrap has been damaged.
- Do not use unless solution is clear and closure is intact.
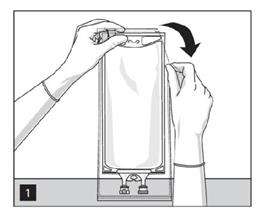
1. To open: Tear overwrap starting from the tear notches. (Figure 1)
2. Prepare medication port by removal of aluminum foil. (Figure 2a)
- Puncture resealable medication port by using 19 – 22 gauge needle and inject additive(s). (Figure 2b)

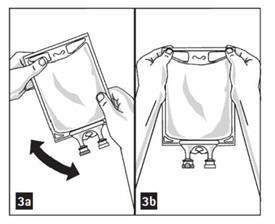
- Mix solution and medication thoroughly. (Figure 3a)
- Medication port must be swabbed with disinfection agent before re-puncturing.
- Check admixture visually for particulate matter. (Figure 3b)
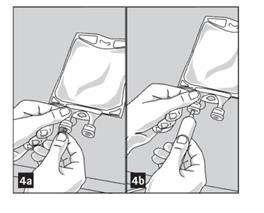
3. Remove aluminum foil from outlet/set port at the bottom of container (Figure 4a) and attach administration set (Figure 4b): use non-vented infusion set or close air vent on a vented set. Refer to directions for use accompanying the administration set.
4. Hang bag on IV Pole. (Figure 5)
B. Braun Medical Inc.
Irvine, CA 92614-5895 USA
1-800-227-2862
www.bbraun.com
Made in USA
Y36-002-823 LD-412-1
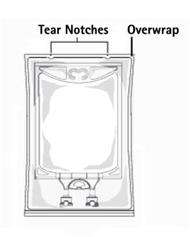
PRINCIPAL DISPLAY PANEL - 3000 mL
1.5% Glycine
Irrigation USP
For Urologic Irrigation
REF R8406
NDC 0264-7390-60
LOT
EXP.
3000 mL
Each 100 mL contains:
Glycine USP 1.5 g
Water for Injection USP qs
pH: 6.1 (4.5-6.5)
Calc. Osmolarity: 200 mOsmol/liter
Not for Injection.
Use only if solution is clear and container and seal are
intact.
Sterile, nonpyrogenic. Single unit container. Discard
unused portion. Use aseptic technique.
Recommended Storage: Room temperature (25°C).
Avoid excessive heat. Protect from freezing.
Do not remove overwrap until ready for use.
See Package Insert.
Not made with natural
rubber latex, PVC or DEHP.
Rx only

SET

B. Braun Medical Inc.
Irvine, CA 92614-5895 USA
1-800-227-2862
www.bbraun.com
Made in USA
Y38-000-011 LD-257-2

GlycineGlycine IRRIGANT
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||